Nontuberculous mycobacterial pulmonary disease – a clinical challenge
Nontuberculous mycobacteria (NTM) are environmental bacteria that can cause pulmonary and extrapulmonary infections. Although pulmonary infection can be severe, isolation of NTM in sputum does not always mean patients will develop disease. Clinical, radiological and microbiological criteria must be met to diagnose NTM pulmonary disease. Treatment is lengthy, requiring multiple antibiotics, and should be guided by a specialist experienced in mycobacterial disease.
- Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and can cause both pulmonary and extrapulmonary infections.
- NTM pulmonary disease (NTM-PD) diagnosis requires the presence of clinical symptoms, specific radiological findings and growth of the same organism in at least two separate sputum cultures (or one culture from a bronchoscopy sample or sterile site in certain circumstances).
- Clinical symptoms include cough, dyspnoea, fever, night sweats, fatigue and haemoptysis but can be variable and nonspecific.
- High-resolution CT imaging is the imaging modality of choice for both diagnosis and monitoring.
- Antimicrobial choice varies depending on the organism and the combination antibiotic regimen should only be given under specialist supervision; treatment courses are lengthy, requiring multiple agents to reduce the emergence of antimicrobial resistance.
- Nonpharmacological management approaches include specialised physiotherapy, aggressive management of coexisting gastro-oesophageal reflux, dietitian advice and smoking cessation.
- All patients with suspected NTM-PD should be referred to a respiratory physician or a specialised mycobacterial clinic if available.
- Prompt referral is very important in the presence of cavitary disease regardless of symptom burden.
Nontuberculous mycobacteria (NTM) are ubiquitous in the environment, including in soil and water.1 The incidence of NTM has increased globally over the past 20 years.2 In Australia, NTM are now isolated more often than Mycobacterium tuberculosis.3 There is variation in the incidence and prevalence across Australia and between other countries, and there are several possible environmental drivers for the geospatial distribution observed. Heavy rainfall, flooding and temperature variation as well as air pollution have been identified as likely contributors to this.4,5
NTM are resistant to common disinfectants and form biofilms allowing them to thrive in household water systems, including filtration devices.1 Furthermore, disinfectants aimed at controlling other waterborne organisms such as Escherichia coli reduce competition for growth and thus may contribute to mycobacterial colonisation.1
NTM are distinct from M. tuberculosis complex and Mycobacterium leprae. Although person to person transmission of Mycobacterium abscessus has been reported among patients with cystic fibrosis, this mode of transmission is considered much less significant for the other NTM species, and no additional isolation or contact tracing is necessarily needed after isolation of NTM in humans.6
There are four main disease manifestations of NTM infection:
- pulmonary disease
- skin and soft tissue infection
- disseminated disease
- lymphadenitis.7
This article focuses on pulmonary disease caused by NTM.
Risk factors
Despite the high prevalence of NTM in the environment, NTM pulmonary disease (NTM-PD) is relatively rare. The estimated incidence rate in Queensland increased 2.3-fold from 11.10/100,000 population in 2001 to 25.88/100,000 in 2016.2 There are several genetic, pre-existing comorbidities and demographic risk factors that make people more vulnerable to disease following exposure.
Cystic fibrosis and primary ciliary dyskinesia are well recognised predisposing factors, and this includes heterozygosity for cystic fibrosis transmembrane receptor (CFTR) mutations.8 Diseases that can result in structural lung damage, such as bronchiectasis, chronic obstructive pulmonary disease and tuberculosis, are also risk factors. Other factors include immunosuppressive states that can develop, for example, in people with advanced HIV infection, transplant recipients or those receiving immunosuppression therapy (particularly with tumour necrosis alpha inhibitors), as well as those with uncommon immunodeficiencies such as adult-onset immunodeficiency with anti-interferon-gamma autoantibodies. Smoking is a significant risk factor and also impairs treatment effectiveness.
A number of people who do not have any of these risk factors can still develop severe disease. Usually, these are postmenopausal women with a typical slender body habitus, of above average height, with hypermobile joints, with varying degrees of pectus excavatum and with a high prevalence of gastro-oesophageal reflux and microaspiration.9 The underlying immunological or genetic defect in this cohort is still unclear but is likely an interplay of multiple different underappreciated factors.10
Diagnosis
The diagnosis of NTM-PD requires the presence of clinical symptoms, specific radiological findings and growth of the same organism in at least two separate sputum cultures (or one culture from a sterile site; see below). Diagnostic criteria for NTM-PD according to the American Thoracic Society, European Respiratory Society, European Society of Clinical Microbiology and Infectious Diseases and Infectious Diseases Society of America clinical practice guideline are summarised in the Table.11
Clinical symptoms can be variable and nonspecific but include cough, dyspnoea, fever, night sweats, fatigue and haemoptysis. In many patients, recurrent bouts of bronchitis, periods of overwhelming fatigue, or both, are the predominant symptoms.
Because it can often be difficult to appreciate significant right middle lobe and lingula disease on plain chest radiography, high resolution CT imaging (HRCT) is the imaging modality of choice for both diagnosis and monitoring.12
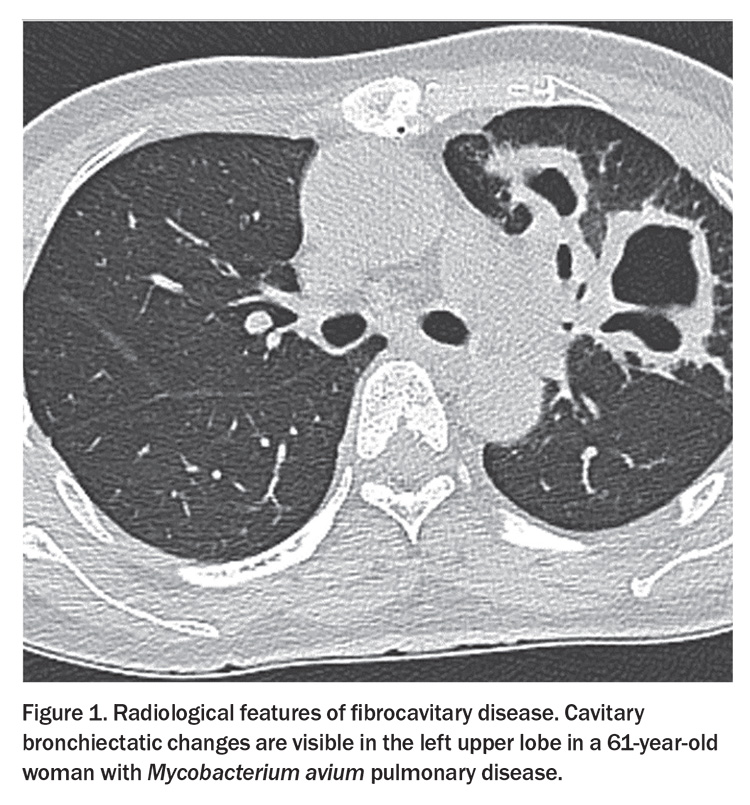
There are two main radiological presentations of NTM-PD: fibrocavitary disease and nodular bronchiectasis (Figure 1 and Figure 2).
Fibrocavitary disease develops as a result of scarring of the lung parenchyma and predominantly affects the upper lobes of the lung. This form of disease carries a worse prognosis and progresses more quickly. It more often affects those with pre-existing structural lung disease, smokers and males.12
In contrast, with nodular bronchiectasis, mucous impaction causes the airways to become damaged and subsequently dilate as often affected patients are unable to effectively clear mucus. This creates an ideal environment for NTM to grow but also perpetuates the issue as thicker secretions become harder to clear contributing to further airway dilatation.13 Patients with nodular bronchiectasis can also develop cavities, a sign indicating more severe and advanced disease.
Microbiological criteria were introduced as NTM can intermittently colonise the lungs without causing infection as well as occasionally contaminate laboratory specimens. Therefore, persistent growth in sputum is key to diagnosis. Many patients cannot produce sputum, and thus in these cases a diagnosis of NTM-PD can be made if NTM are cultured once from bronchoalveolar lavage fluid in someone who otherwise meets the clinical and radiological criteria. When NTM-PD is suspected, sputum should be sent for mycobacterial culture (an acid-fast bacilli [AFB] culture), as different laboratory methods are used for this test compared with those used to process sputum sent for standard bacterial microscopy culture and sensitivity (MCS).
There are over 200 different species of NTM, but they are not equal in regard to their pathogenicity. For instance, Mycobacterium gordonae rarely causes human pulmonary disease as opposed to Mycobacterium kansasii or M. abscessus.14 In Australia, the NTM species that are most often reported are Mycobacterium intracellulare, Mycobacterium avium, M. abscessus and Mycobacterium fortuitum.15
Management
The management of NTM-PD is divided into pharmacological and nonpharmacological approaches.
Many clinicians are reluctant to treat NTM-PD as they feel treatment side effects outweigh the benefits of treatment. However, most patients will have a significant improvement in their symptoms with treatment and, with appropriate monitoring, most side effects are manageable. Although a period of observation is often warranted, excessive delay in treatment when indicated can result in prolonged symptoms and progression of disease with a subsequent lower likelihood of cure.
Antimicrobial choice varies depending on the organism and should only be given under specialist supervision. The antimicrobials used to treat NTM-PD are only available via special/compassionate access schemes or off-label. The treatment courses are lengthy and require multiple agents used together. Usually, antimicrobials are continued for 12 months after the first of at least three consecutive negative sputum cultures collected monthly (referred to as culture conversion).
A commonly used treatment combination for M. avium or M. intracellulare infection is azithromycin, ethambutol and rifampicin. Intravenous amikacin may be used initially in severe disease with nebulised amikacin or oral clofazimine being added in cases of refractory disease or drug intolerance. Macrolide monotherapy should never be used for NTM treatment as it may result in macrolide resistance and incurable disease.
The treatment for M. abscessus usually starts with an intravenous treatment induction for four to six weeks (usually with amikacin, imipenem and tigecycline) plus oral azithromycin/clarithromycin and clofazimine, followed by a consolidation phase (usually with three different antibiotics). Possible choices based on susceptibility testing include azithromycin, rifabutin, clofazimine, inhaled amikacin, linezolid, doxycycline, moxifloxacin or bedaquiline.
In selective cases there is also a role for surgical resection. This may be considered, for example, in patients with a localised cavity who are not responding to medical therapy.13
In addition to antimicrobials, patients should receive specialised physiotherapy instruction in airway clearance. In addition to active cycle breathing and huffing techniques, the use of an oscillatory positive expiratory pressure device or nebulised hypertonic saline, or both, may be recommended. Aggressive management of coexisting gastro-oesophageal reflux is also essential as this is a potential mechanism for repeat infection. This not only includes the use of proton pump inhibitors but also using a wedge pillow and avoidance of late-night meals.
As so many patients with NTM-PD are underweight, early involvement of a dietitian can be very useful to ensure patients are following a high-energy, high protein diet. A body mass index less than 18.5 kg/m2 portends a poor prognosis.16,17
Ongoing smoking makes it extremely difficult to achieve cure in NTM-PD due to the impairment of macrophage function.18 The importance of smoking cessation must be emphasised at every review for patients who continue to smoke.
Reducing exposure to NTM is also crucial to prevent reinfection. Given the possibility of potable water and soil as a source of re-exposure, it may be advisable for some patients to boil their drinking water, ensure hot water systems are set at a minimum of 60°C and wear a mask when gardening. Filtration water devices and plumbed-in fridges often harbour NTM and should be avoided.
Monitoring during treatment
In the first instance if NTM are isolated in sputum, patients should be evaluated for clinical symptoms of NTM-PD and submit more sputum samples to see if there is persistent growth with the same pathogen. All patients should have a HRCT scan to look for radiological features of NTM-PD.
While on treatment, patients will be under close clinical surveillance but are also asked to regularly submit more sputum samples (usually monthly). This is important to be able to document the date of culture conversion, identify refractory disease and identify any other co-existing pathogens. Repeat imaging with HRCT is usually performed towards the end of treatment to evaluate the radiological response.
Several potential adverse drug reactions are associated with NTM treatment. One of the more common concerns is the risk of optic neuropathy secondary to ethambutol treatment, which is an important component of regimens used to treat M. avium and M. intracellulare pulmonary disease. The risk is about 1% with standard 15 mg/kg daily dosing.19 The risk increases with higher doses, increased age, lower body mass index and associated renal disease and in smokers. Patients should be assessed by their optometrist and/or ophthalmologist before the start of therapy to document a baseline visual acuity and any pre-existing eye disease. Regular formal assessments are of questionable cost-efficacy. Rather, patients should be questioned about their vision and if they report changes (including colour vision changes), they should be re-evaluated urgently by an optometrist or ophthalmologist to determine if these are related to ethambutol. If there is likely to be a delay in assessment, ethambutol should be ceased pending this assessment and the patient’s treating specialist informed.20
The most common side effect of rifampicin is a flu-like illness with liver enzyme derangement. It can also cause leucopenia. Initial blood test monitoring usually identifies this problem early in the treatment course. However, beyond the first two months of treatment, blood tests are only performed for specific clinical reasons.
It is also important to be aware that rifampicin is a potent inducer of CYP3A4 and, therefore, the potential for drug–drug interactions (most often with anticoagulants, thyroxine, prednisolone, hormone preparations, HMG-CoA reductase inhibitors) must always be checked before its initiation. Patients should be advised not to start any other new medications without first discussing this with their GP or treating specialist to ensure an up-to-date interactions check can be completed.20 In patients with multiple comorbidities and concomitant medications, a review by a clinical pharmacist may be useful.
Azithromycin can prolong the QTc interval on the ECG. This is usually only clinically important in patients who have underlying cardiac disease or who are on additional medications that may also prolong the QTc, in which case ECG monitoring is often performed. This effect should also be considered when prescribing other therapies that may add to this effect (e.g. sotalol, amiodarone, domperidone, ciprofloxacin and azole antifungals).
When to refer
All patients who are suspected to have NTM-PD should be referred to a respiratory physician or a specialised mycobacterial clinic if available. This should include patients with clinical symptoms of NTM-PD and nodular bronchiectasis or cavities on imaging, with or without growth of NTM in sputum cultures. The presence of cavities should result in an urgent referral and review given the risk of disease progression and poorer prognosis. It is useful to organise repeat sputum sample collection with the referral as well as cross- sectional imaging with HRCT if not already performed.
All patients with NTM-PD should have lifelong follow up as relapses and reinfection from environmental sources are common.
Conclusion
It is important to be aware of the potential severity of pulmonary infection that NTM can cause and, if suspected, to organise sputum samples to be sent for mycobacterial culture as well as arranging cross-sectional imaging, ideally with a HRCT. Prompt referral of patients is essential if cavities are present, but all patients with suspected or confirmed NTM-PD should ideally be seen by specialists experienced in treating mycobacterial infection. RMT
COMPETING INTERESTS: Dr Grey: None. Professor Thomson has received consulting fees for her institution from AN2 Therapeutics; honoraria for speaking at Korean NTM meeting, Seoul National University Hospital; partial travel expenses to speak at the World Bronchiectasis Congress, New York; financial support to attend CHEST meeting in Nashville and present trial results for Beyond Air; and unpaid positions as Chair of the Pulmonary Infections and Tuberculosis Assembly and Nominating Committee, and Board Member of the American Thoracic Society (ATS) and ATS Membership Committee.
References
1. Falkinham JO, 3rd. Ecology of nontuberculous mycobacteria. Microorganisms 2021; 9(11): 2262.
2. Ratnatunga CN, Lutzky VP, Kupz A, et al. The rise of non-tuberculosis mycobacterial lung disease. Front Immunol 2020; 11: 303.
3. Chou MP, Clements ACA, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis 2014; 14: 279.
4. Thomson RM, Furuya-Kanamori L, Coffey C, Bell SC, Knibbs LD, Lau CL. Influence of climate variables on the rising incidence of nontuberculous mycobacterial (NTM) infections in Queensland, Australia 2001-2016. Sci Total Environ 2020; 740: 139796.
5. Hamed KA, Tillotson G. A narrative review of nontuberculous mycobacterial pulmonary disease: microbiology, epidemiology, diagnosis, and management challenges. Expert Rev Respir Med 2023; 17: 973-988.
6. Bryant JM, Grogono DM, Greaves D, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 2013; 381: 1551-1560.
7. Ford TJ, Silcock RA, Holland SM. Overview of nontuberculous mycobacterial disease in children. J Paediatr Child Health 2021; 57: 15-18.
8. Polgreen PM, Comellas AP. Clinical phenotypes of cystic fibrosis carriers. Annu Rev Med 2022; 73: 563-574.
9. Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178: 1066-1074.
10. Koh W-J. Nontuberculous mycobacteria—overview. Microbiol Spectr 2017; 5(1): 10.1128/microbiolspec.tnmi7-0024-2016.
11. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020; 71(4): e1-e36.
12. Musaddaq B, Cleverley JR. Diagnosis of non-tuberculous mycobacterial pulmonary disease (NTM-PD): modern challenges. Br J Radiol 2020; 93(1106): 20190768.
13. Pathak K, Hart S, Lande L. Nontuberculous mycobacteria lung disease (NTM-LD): current recommendations on diagnosis, treatment, and patient management. Int J Gen Med 2022; 15: 7619-7629.
14. Matsumoto Y, Kinjo T, Motooka D, et al. Comprehensive subspecies identification of 175 nontuberculous mycobacteria species based on 7547 genomic profiles. Emerg Microbes Infect 2019; 8: 1043-1053.
15. Nontuberculous mycobacteria in Queensland. Queensland Health; 2016. Available online at https://www.health.qld.gov.au/__data/assets/pdf_file/0022/660145/report-tb-ntm-2016.pdf (accessed March 2024).
16. Sadamatsu H, Takahashi K, Tashiro H, et al. A low body mass index is associated with unsuccessful treatment in patients with Mycobacterium avium complex pulmonary disease. J Clin Med 2021; 10: 1576.
17. Tanaka G, Jo T, Tamiya H, Sakamoto Y, et al. Factors affecting in-hospital mortality of non-tuberculous mycobacterial pulmonary disease. BMC Infect Dis 2021; 21: 698.
18. Charles SH, John B, Toby C, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017; 72(Suppl 2): ii1-ii64.
19. Saxena R, Singh D, Phuljhele S, et al. Ethambutol toxicity: expert panel consensus for the primary prevention, diagnosis and management of ethambutol-induced optic neuropathy. Indian J Ophthalmol 2021; 69: 3734-3739.
20. Sawka A, Burke A. Medications and monitoring in treatment of nontuberculous mycobacterial pulmonary disease. Clinics Chest Med 2023; 44: 815-828.