Chronic thromboembolic pulmonary hypertension: an overview

Chronic thromboembolic pulmonary hypertension is a rare cause of dyspnoea that should be suspected in any patient with persistent breathlessness after pulmonary embolism. After initial investigations to confirm pulmonary hypertension and pulmonary embolism, investigation and management at an expert centre are recommended, as treatment is potentially curative.
- Although rare, chronic thromboembolic pulmonary hypertension (CTEPH) should be considered in any patient with persistent breathlessness after a diagnosis of pulmonary embolism.
- Diagnosis includes confirmation of chronic emboli on ventilation-perfusion scanning and CT pulmonary angiography after at least three months of effective anticoagulation therapy, with pulmonary hypertension evident on transthoracic echocardiogram and confirmed on right heart catheterisation.
- Ventilation-perfusion scanning is the most effective screening tool to exclude CTEPH, as CT pulmonary angiography may miss distal disease.
- Pulmonary endarterectomy is potentially curative in appropriate patients with operable disease and is considered the first-line therapy.
- Balloon pulmonary angioplasty and medical therapy are offered to patients with nonoperable disease.
- Lifelong anticoagulation therapy is essential for all patients, in the absence of an absolute contraindication to anticoagulation.
- Investigation and management at an expert CTEPH centre are recommended.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare progressive pulmonary vascular disease that develops in some patients after acute pulmonary embolism (PE). CTEPH should be considered in any patient with persistent breathlessness (especially after a diagnosis of PE) or as a differential diagnosis in the workup of pulmonary hypertension (PH). Its management differs to that of acute PE and other causes of PH. Importantly, treatment is potentially curative.
The natural history of most cases of acute PE is complete thrombus resolution, aided by intrinsic thrombolytic mechanisms, with anticoagulation therapy reducing further thrombus production. However, in a small proportion of patients, there is a failure of thrombus clearance despite appropriate treatment, leading to chronic organised fibrotic clot material that obstructs the pulmonary arteries. This clot material is rich in collagen and elastin, with partial recanalisation and, in some, with calcification. Organised clot is a consequence of and potentiates altered vascular remodelling, impaired angiogenesis and fibrinolysis, and endothelial dysfunction.1 This leads to a secondary vasculopathy, further increasing pulmonary vascular resistance, with the development of PH and, ultimately, right ventricular failure.1
How common is CTEPH?
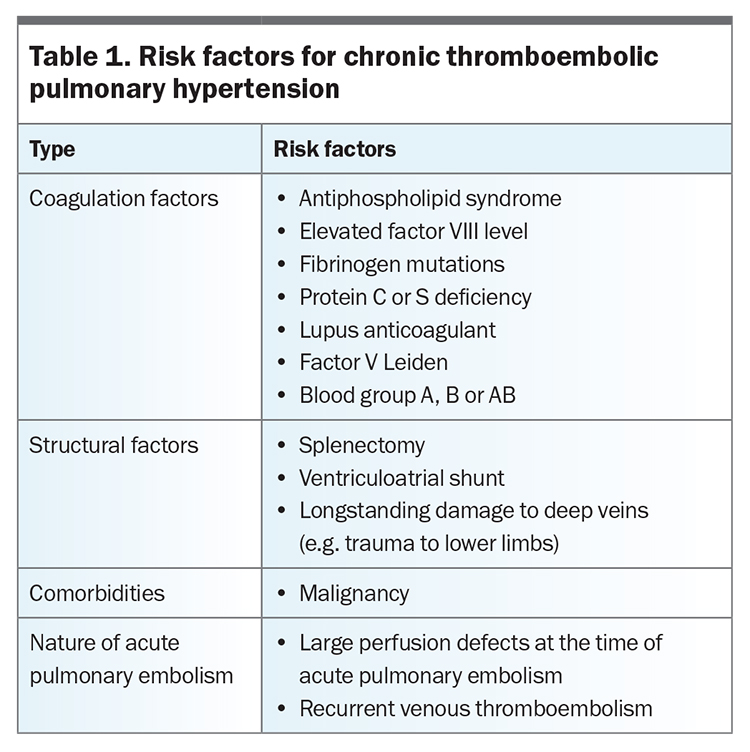
The true incidence of CTEPH in the general population is unknown but is estimated at five per one million adults annually, although it is more common in those with a history of acute PE and persistent dyspnoea (5 to 8%).2,3 There are no clear data to support routine screening of patients for CTEPH after PE, but the diagnosis should be considered in those with persistent breathlessness after PE. A quarter of patients with CTEPH have no documented history of acute PE (or compatible clinical presentation).4 Table 1 presents risk factors for the development of CTEPH.5
Clinical presentation
Patients present with insidiously progressive dyspnoea, fatigue, reduced exercise tolerance and, in the late stages of disease, right heart failure, peripheral oedema, chest pain and syncope. Patients often report a ‘honeymoon period’ (which may last months or even years) of initial reduction in symptoms after the diagnosis of acute PE, followed by worsening symptoms.
Investigation
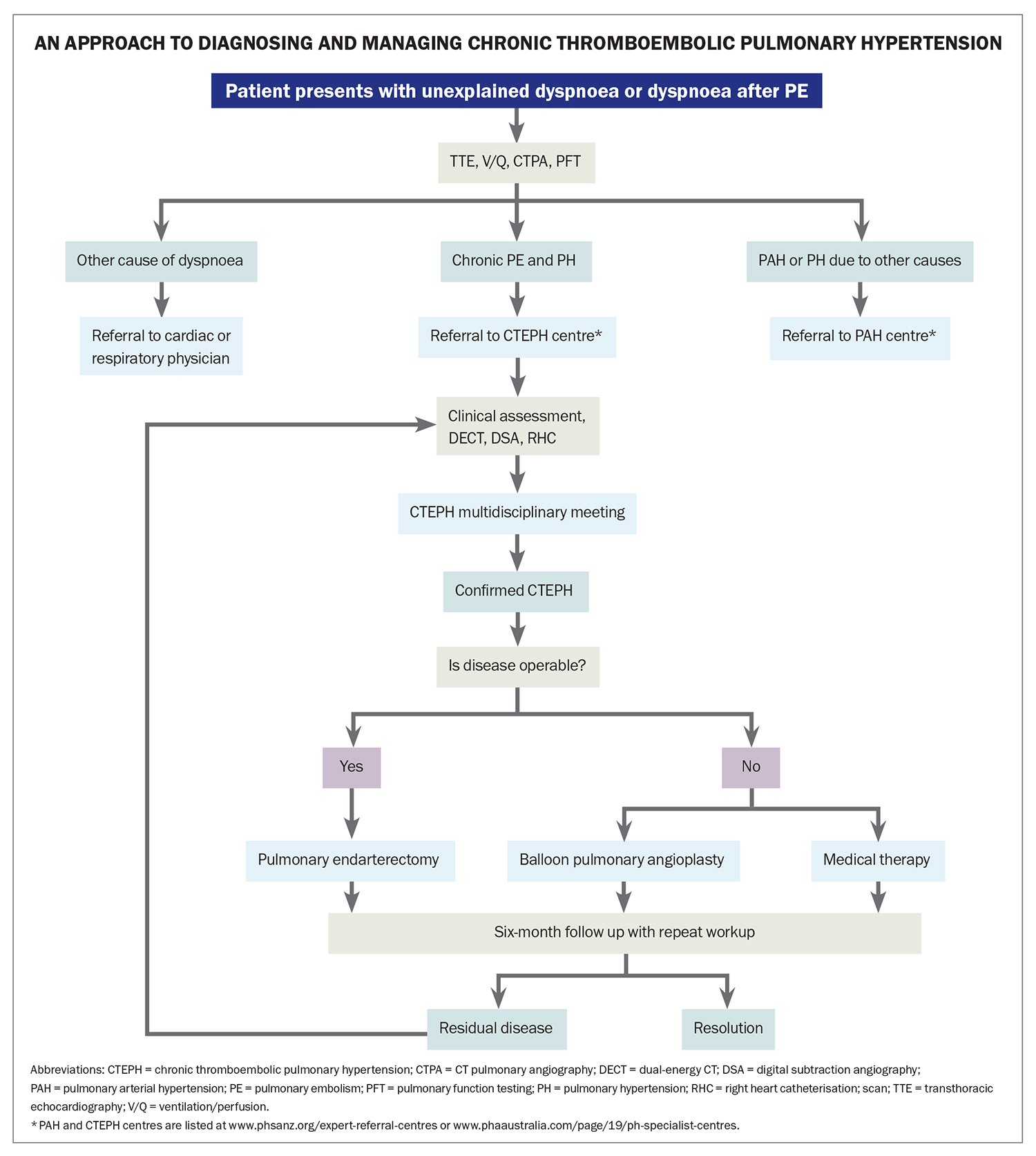
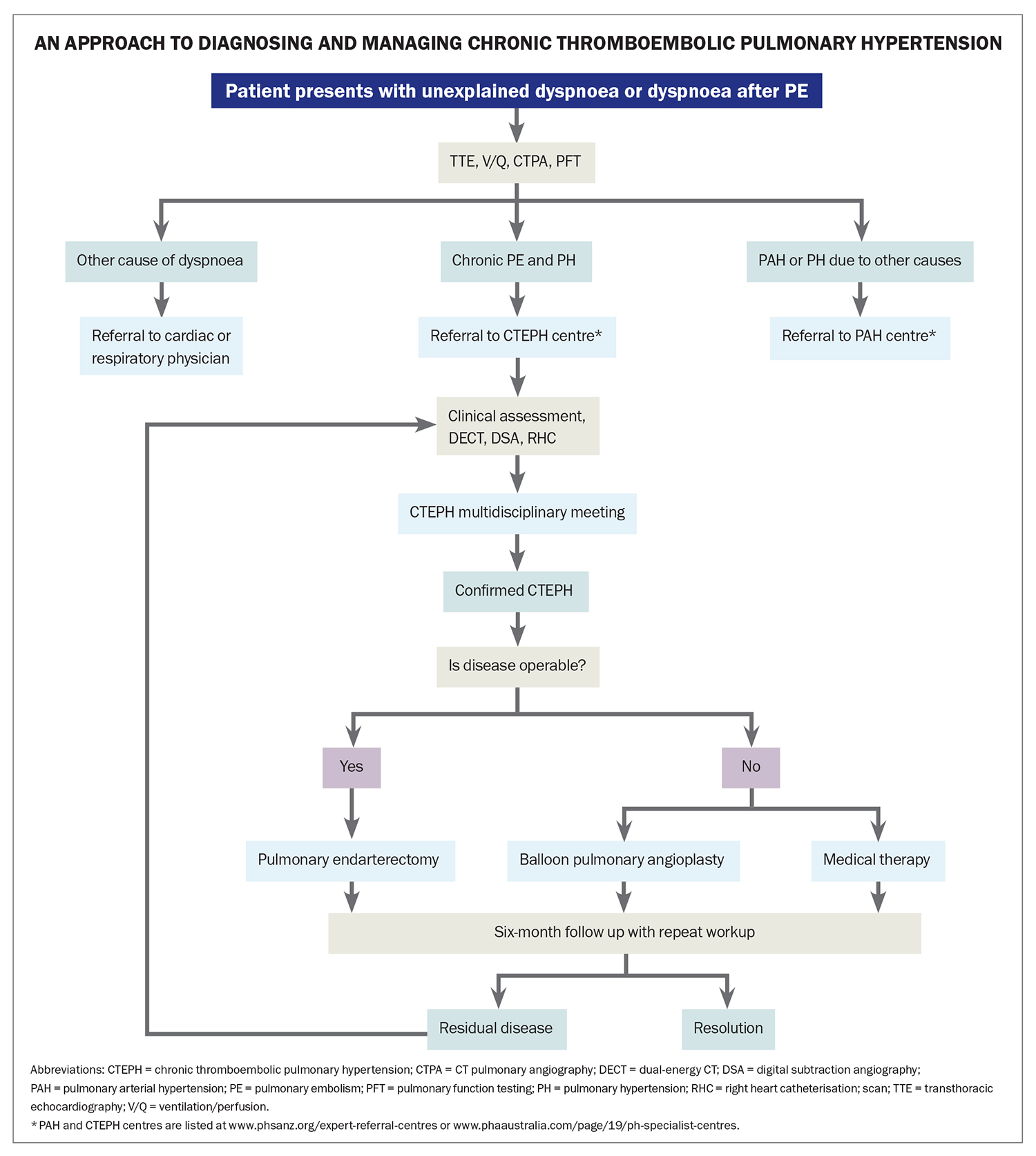
The diagnosis of CTEPH includes confirmation of chronic thromboembolic obstruction of the pulmonary vascular bed (despite three months of therapeutic anticoagulation), assessment of cardiac haemodynamic parameters, measures of functional limitation and consideration of comorbidities (Flowchart). Given the rarity and complexity of this disease, after initial investigations to confirm PH and PE, referral to a specialist centre experienced in caring for patients with CTEPH is recommended for further investigation and management, as recommended by international guidelines.6 Expert centres are listed at www.phsanz.org/expert-referral-centres/ or www.phaaustralia.com/page/19/ph-specialist-centres.
Imaging
Ventilation-perfusion (V/Q) scanning is the best test to exclude chronic thrombotic obstruction of the pulmonary vascular bed. In patients with CTEPH, there is absent perfusion in lung segments that correspond with intact ventilation (V/Q mismatch). V/Q scans provide superior sensitivity (90 to 100%) compared with CT pulmonary angiography (CTPA) (51 to 99%).7,8
Although V/Q scans are more sensitive in detecting PE, CTPA helps with defining the location and extent of fibrotic clot burden, which informs the assessment of surgical removal. In contrast to acute PE, where the clot is located centrally in the vascular lumen and causes partial or complete occlusion, chronic thromboemboli are usually eccentric, contiguous with the vessel wall, and recanalised fibrotic embolic material leads to the appearance of webs, bands and stenoses. Enlargement of the pulmonary trunk may also be seen.9 Indirect features of CTEPH seen on CTPA include mosaic attenuation of the lung parenchyma, due to geographical variation in perfusion, and enlarged collateral bronchial arteries.10
Subsegmental disease is better appreciated on dual-energy CT, which enables functional assessment of lung perfusion by generating pulmonary blood volume maps from iodine distribution.11 Catheter pulmonary angiography (using digital subtraction angiography, with one to two images per second for at least six seconds after injection) provides valuable information regarding the location of thrombi and is superior to other noninvasive imaging modalities in depicting subsegmental disease. Relative regional flow can be assessed where incomplete obstruction is present. Catheter pulmonary angiography is usually only available in expert referral centres, where it is best performed. It is often undertaken to assess whether the disease is amenable to pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA).
Cardiopulmonary haemodynamics
Transthoracic echocardiography is a useful investigation to screen for PH. It also provides key information about the structure and function of the right heart; the failing heart may show right ventricular dilatation and dysfunction.
Right heart catheterisation is then required to confirm PH and to access PBS-funded therapy. Elevation in mean pulmonary artery pressure (mPAP >20 mmHg) and pulmonary vascular resistance (PVR ≥2 Woods units) confirms PH.6,12 Right heart catheterisation can also show any contribution from left heart disease (elevated pulmonary capillary wedge pressure) and measure severity (PVR and cardiac index). Severe elevation of PVR (≥12 Woods units) before surgical intervention, and incomplete resolution after surgery, is a poor prognostic marker.13 Right heart catheterisation should only be performed in experienced centres to ensure patient safety and complete and accurate data collection.
Additional tests
In patients with CTEPH, pulmonary function tests may show mild reductions in forced vital capacity and forced expiratory volume, thought to be related to scarring following lung infarction.14 Diffusing capacity for carbon monoxide is often reduced and is thought to indicate the degree of microvasculopathy in patients with CTEPH.15 The six-minute walk test is a useful objective measure of functional capacity, disease progression and treatment response. Cardiopulmonary exercise testing often shows an impaired aerobic exercise capacity, decreased peak oxygen uptake and ventilatory inefficiency caused by increased dead space ventilation (reflected by an elevated minute ventilation/carbon dioxide production ratio and low end-tidal partial pressure of carbon dioxide).16
In addition to cardiopulmonary exercise testing, symptomatic patients with chronic thromboembolic disease without PH at rest (CTED) are often assessed with exercise right heart catheterisation to identify exercise-induced PH. Identification of ventilatory inefficiency and exercise-induced PH is pivotal to the risk-benefit assessment when considering surgery for patients with CTED.17
Differential diagnoses
Differential diagnoses of CTEPH include CTED, PH from another cause and nonthrombotic obstruction of the pulmonary vascular bed (e.g. angiosarcoma and sarcoidosis). Disease ‘progression’ is often incorrectly attributed to recurrent acute emboli but is more likely related to progressive arteriopathy. Recurrent PE should lead to an assessment of compliance with, or appropriateness of, the anticoagulation regimen, and screening for a procoagulant state should also be considered.
Management
Lifelong anticoagulation therapy is recommended for all patients with CTEPH to prevent recurrent venous thromboembolism. Warfarin is the recommended agent. This is especially so for patients who have undergone PEA, among whom the rate of venous thromboembolism recurrence appears much higher in those treated with direct oral anticoagulants (4.62%/person-year) compared with vitamin K antagonists (0.76%/person-year), with equivalent major bleeding risks (based on retrospective registry data).18 Warfarin or low molecular weight heparin should also be used for patients at high risk of thrombosis (e.g. those with antiphospholipid syndrome).19 Oxygen supplementation should be offered to patients with hypoxaemia, and pulmonary rehabilitation is recommended for all patients to improve exercise tolerance.
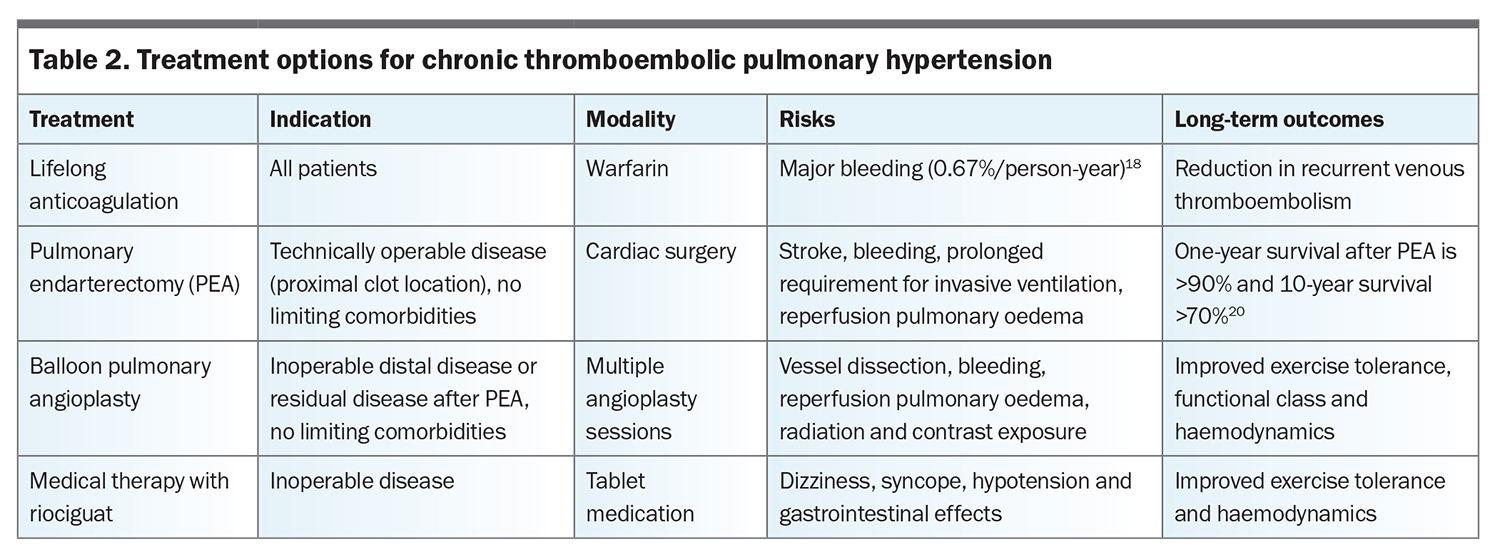
Choice of further treatment is determined by technical factors (particularly proximal extent and accessibility of the obstruction), functional limitation, impact on prognosis and patient comorbidities and preferences (Flowchart). Treatment options for CTEPH are summarised in Table 2. Multiple factors are considered when assessing suitability for surgical intervention. The recommended approach is to present a detailed relevant clinical history and results of investigations to a CTEPH multidisciplinary meeting to confirm the diagnosis and guide management. The multidisciplinary team should include a cardiothoracic surgeon experienced in PEA, a PH specialist, a radiologist experienced in CTEPH and an interventionist experienced in BPA. Key factors guiding treatment choices include the location of thromboembolic disease and its accessibility for surgical intervention, comorbidities that might complicate recovery from surgery and patient preferences.
Pulmonary endarterectomy
The benefits versus risks of surgical intervention differ based on whether patients have PH (i.e. CTEPH) or no PH (i.e. CTED). It is known that PEA confers a survival advantage for patients with CTEPH.20,21 However, there is insufficient evidence to determine if CTED will progress to CTEPH, and whether PEA improves the prognosis for patients with CTED. Although there have been reports of symptomatic and functional improvements from PEA for patients with CTED, this should only be considered at centres with significant expertise. The risk-benefit assessment needs to take into account the degree of increased dead space ventilation and exercise-induced PH, as well as a thorough evaluation for alternative causes of exertional dyspnoea.17
For appropriately chosen patients, PEA offers the best chance of symptomatic and prognostic improvement and is potentially curative. PEA should be offered upfront for patients with operable disease, rather than considering BPA or medical therapy as first-line treatment. To be considered technically operable, thromboembolic disease should be located in the main lobar or segmental arteries. Comorbidities that should be considered include significant parenchymal lung disease, which may be worsened by exacerbating V/Q mismatch, and dyspnoea predominantly from other causes, such as left heart disease, for which PEA is unlikely to confer significant benefit.
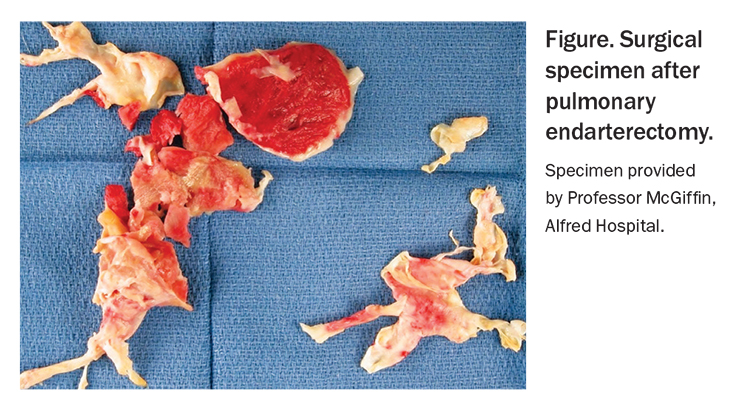
PEA requires specialist training for both the surgical and postoperative aspects of care. During surgery, a median sternotomy is performed, followed by cardiopulmonary bypass, cooling (to 20°C) and circulatory arrest. Deep hypothermic circulatory arrest is limited to 20-minute intervals, during which the fibrotic thromboembolic material is dissected away from the vessel wall (Figure). Successful surgery usually results in immediate improvement in PAP and PVR, and three-quarters of patients achieve near-complete normalisation of pulmonary haemodynamics.22
PEA is a complex surgery, and complications include stroke, bleeding, infection and a prolonged requirement for invasive ventilation. Reperfusion pulmonary oedema is a common postoperative event and results in hypoxaemia and radiological evidence of pulmonary infiltrates in areas of reperfused lung. Specialist postoperative care can improve outcomes, and some patients leave hospital with supplemental oxygen therapy, from which they are weaned shortly thereafter. In-hospital mortality is relative to the centre’s experience and is about 2 to 5%.20 One-year survival after PEA is greater than 90%, and 10-year survival is greater than 70%.20 This is compared with five-year survival of 10% in patients with untreated CTEPH.21
Balloon pulmonary angioplasty
For those with inoperable distal disease or residual disease after PEA, BPA is emerging as an effective treatment option. During BPA, a guidewire is threaded into the femoral or jugular vein and undersized balloons are expanded over the guidewire, with the aim of breaking up intraluminal webs and bands. Repeat sessions (e.g. four to 10 sessions two to six weeks apart) are often required. BPA should only be performed by experienced specialists in expert centres. Complications include vessel dissection and pulmonary parenchymal bleeding from vascular injury. Additional risks include repeated radiation and contrast exposure, with the attendant risk of contrast-induced nephropathy. Compared with medical therapy, BPA improves New York Heart Association functional class, six-minute walk distance (6MWD) and haemodynamic parameters for patients with inoperable CTEPH.23
Medical therapy
For patients with inoperable CTEPH or residual PH after PEA, medical therapy is offered, at times in conjunction with BPA. Riociguat is a soluble guanylate cyclase stimulator, which results in vasorelaxation and antiproliferative effects. The CHEST-1 study (A Study to Evaluate Efficacy and Safety of Oral BAY63-2521 in Patients with CTEPH) was a phase 3 randomised placebo-controlled trial of riociguat for patients with either inoperable CTEPH or persistent or recurrent PH after PEA. It showed an increase in 6MWD and improvement in haemodynamics at 16 weeks for patients treated with riociguat.24 Common side effects of riociguat include dizziness, syncope, hypotension and gastrointestinal effects. Riociguat is started at a low dose of 1 mg three times daily and uptitrated to the highest tolerated dose, to a maximum of 2.5 mg three times daily.
Pulmonary arterial hypertension-specific therapy, with sildenafil (a phosphodiesterase-5 inhibitor that promotes smooth muscle relaxation in the lung vasculature), bosentan and macitentan (endothelin receptor antagonists that inhibit lung vessel constriction), has also been trialled in patients with CTEPH, with some benefit seen.25-27 As these agents have not been studied in large randomised controlled trials, riociguat is the only PBS-listed medical therapy in Australia. To be eligible for riociguat, patients must have inoperable disease (as assessed by a centre with expertise in managing CTEPH) or have persistent PH six months after PEA. Riociguat must be prescribed by a pulmonary arterial hypertension specialist. Patients who cannot tolerate riociguat because of side effects should be referred to an expert centre for consideration of other therapeutic options.
Regular follow-up is required, with a clinical review, 6MWD testing and transthoracic echocardiography at least every six months. Pulmonary rehabilitation should be offered yearly until patients return to their baseline level of function. When resolution of PH is achieved after PEA, follow-up may be performed yearly or every two years. In some cases, shared care between the expert centre and the patient’s treating respiratory physician or cardiologist is appropriate. To enable the patient’s GP to provide good ongoing support, the expert centre should clearly communicate to the GP the reason for the choice of treatments, possible side effects of therapy and the expectations of improvement in each individual case.
Conclusion
CTEPH is a rare cause of breathlessness after PE, but diagnosing it is important because it may be cured surgically with PEA. As such, a ‘surgery first’ philosophy is adopted, as this is associated with substantially improved survival. Most, but not all, patients have a history suggestive of past PE. Unresolved symptoms three months after an acute PE warrant further evaluation for CTEPH. Lifelong anticoagulation therapy is indicated for all patients with CTEPH. Major decisions about investigation and management of patients with (or suspected to have) CTEPH or CTED should be made at an expert centre. RMT
COMPETING INTERESTS: Dr Barnes has received travel support from Janssen, United Therapeutics and Boehringer Ingelheim. Dr Yo and Professor Williams: None.
References
1. Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160112.
2. Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49: 1601792.
3. Rådegran G, Kjellström B, Ekmehag B, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J 2016; 50: 243-250.
4. Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973-1981.
5. Delcroix M, Kerr K, Fedullo P. Chronic thromboembolic pulmonary hypertension. Epidemiology and risk factors. Ann Am Thorac Soc 2016; 13 Suppl 3: S201-S206.
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618-3731.
7. Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48: 680-684.
8. He J, Fang W, Lv B, et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun 2012; 33: 459-463.
9. Ruggiero A, Screaton NJ. Imaging of acute and chronic thromboembolic disease: state of the art. Clin Radiol 2017; 72: 375-388.
10. Ley S, Ley-Zaporozhan J, Pitton MB, et al. Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol 2012; 22: 607-616.
11. Renard B, Remy-Jardin M, Santangelo T, et al. Dual-energy CT angiography of chronic thromboembolic disease: can it help recognize links between the severity of pulmonary arterial obstruction and perfusion defects? Eur J Radiol 2011; 79: 467-472.
12. Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915.
13. Skoro-Sajer N, Marta G, Gerges C, et al. Surgical specimens, haemodynamics and long-term outcomes after pulmonary endarterectomy. Thorax 2014; 69: 116-122.
14. Morris TA, Auger WR, Ysrael MZ, et al. Parenchymal scarring is associated with restrictive spirometric defects in patients with chronic thromboembolic pulmonary hypertension. Chest 1996; 110: 399-403.
15. Suda R, Tanabe N, Ishida K, et al. Prognostic and pathophysiological marker for patients with chronic thromboembolic pulmonary hypertension: usefulness of diffusing capacity for carbon monoxide at diagnosis. Respirology 2017; 22: 179-186.
16. Held M, Grün M, Holl R, et al. Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respiration 2014; 87: 379-387.
17. McGuire WC, Alotaibi M, Morris TA, Kim NH, Fernandes TM. Chronic thromboembolic disease: epidemiology, assessment with invasive cardiopulmonary exercise testing, and options for management. Structural Heart 2021; 5: 120-127.
18. Bunclark K, Newnham M, Chiu Y-D, et al. A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J Thromb Haemost 2020; 18: 114-122.
19. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018; 132: 1365-1371.
20. Jenkins D, Madani M, Fadel E, Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111.
21. Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151-158.
22. Hsieh WC, Jansa P, Huang WC, Nižnanský M, Omara M, Lindner J. Residual pulmonary hypertension after pulmonary endarterectomy: a meta-analysis. J Thorac Cardiovasc Surg 2018; 156: 1275-1287.
23. Kalra R, Duval S, Thenappan T, et al. Comparison of balloon pulmonary angioplasty and pulmonary vasodilators for inoperable chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Sci Rep 2020; 10: 8870.
24. Ghofrani H-A, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319-329.
25. Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229-236.
26. Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008; 52: 2127-2134.
27. Ghofrani HA, Simonneau G, D’Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 2017; 5: 785-794.