Managing asthma in pregnancy: new research and resources for GPs

Asthma is the most prevalent chronic disease affecting pregnant women. Many women will be managed by their GP at some point during their pregnancy. The GP plays a pivotal role in educating patients and assessing and managing risk factors for asthma exacerbation during pregnancy, which can help optimise asthma management and health outcomes for the mother and child.
- Women with asthma are at increased risk of poor perinatal outcomes, including preterm birth, low birth weight and infant congenital malformations.
- Asthma medications are safe to use throughout pregnancy and women should be encouraged to continue using their prescribed asthma medications throughout pregnancy and postpartum.
- Modifiable risk factors, such as smoking and depression and anxiety, and nonmodifiable risk factors, including multiparity and multiple pregnancy, are associated with worsening asthma symptoms and exacerbations, and should be targeted for management.
- Educating pregnant women about the safety and correct use of their asthma medications and the importance of maintaining good asthma control during pregnancy is essential for improving outcomes for women with asthma during pregnancy.
- Recognising patient characteristics associated with poor adherence, such as lower maternal age, higher parity or being a current or ex-smoker, can help clinicians identify those who may need further education on asthma medication in pregnancy.
- The Asthma in Pregnancy Toolkit provides information and resources, including infographics, webinars and links to relevant guidelines and clinical assessments for health professionals and families.
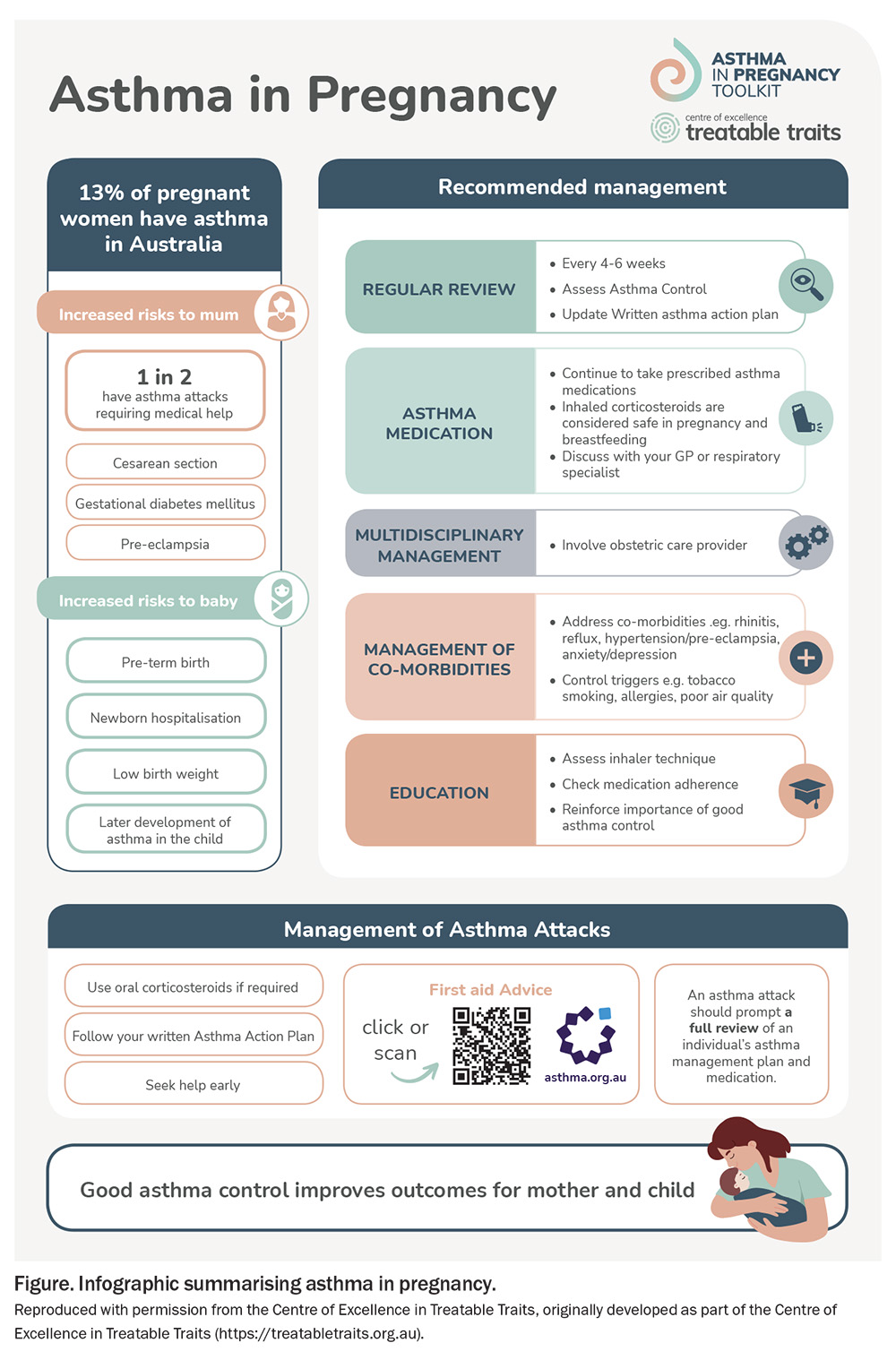
Asthma affects more than one in 10 people in Australia, and recent studies show that between 8.4% (South Australia) and 17% (Queensland) of pregnancies are affected, making this an important issue for GPs.1,2 This article outlines key new research findings that will assist clinicians in optimising asthma management for their pregnant patients. We describe risk factors for exacerbation of asthma in pregnancy, important aspects of self-management that GPs can help improve, and the potential for a ‘treatable traits’ approach to asthma management in pregnancy. New resources are also available for health professionals and families affected by asthma in pregnancy on the Asthma in Pregnancy Toolkit website (https://asthmapregnancytoolkit.org.au), which covers key aspects of asthma management in pregnancy (Figure).
Asthma medication use in pregnancy
Asthma medication is generally considered safe to continue during pregnancy. Although randomised controlled trials are limited (as with any medication use in pregnant women), an abundance of data supports the safety of short-acting beta agonists (SABAs), particularly salbutamol, and inhaled corticosteroids (ICS), particularly budesonide.3,4 Women whose asthma has been well controlled on a particular ICS are recommended to continue this ICS in pregnancy. The benefit of oral corticosteroids (OCS) in treating an exacerbation is considered to outweigh any potential fetal risk. Fewer data are available on the safety of biologics; however, registry studies have been established and are currently collecting data. Published data on omalizumab show a similar rate of poor outcomes (stillbirth, low birth weight, preterm birth and congenital malformations) in pregnant women with asthma who were exposed to omalizumab compared with an unexposed cohort.5
Perinatal and neonatal outcomes
Pregnant women with asthma are at increased risk of poor perinatal outcomes. Consequently, asthma management in pregnancy is important because it has the potential to improve outcomes for both the mother and baby. Our team recently updated our systematic review and meta-analysis examining the neonatal risks of asthma in pregnancy, including the addition of 18 new studies and more than 15 million pregnancies to the analysis (including >600,000 with asthma), with the following reported outcomes.6
- Most risks remained as previously described: infants of women with asthma were more likely to go to the neonatal intensive care unit (NICU) or special care nursery (relative risk [RR], 1.27; 95% confidence interval [CI], 1.25–1.30) and have congenital malformations (RR, 1.36; 95% CI 1.32–1.40).7 These results were supported by combining data from studies that adjusted for important confounders.
- The risk for major congenital malformations became statistically significant (RR, 1.18; 95% CI, 1.15–1.21; adjusted odds ratio [aOR], 1.20; 95% CI, 1.15–1.26).
- The risk for respiratory distress syndrome became statistically significant (RR, 1.25; 95% CI, 1.17–1.34).
- Neonatal death was no longer significantly associated with maternal asthma – although perinatal mortality reached significance in the unadjusted analysis, this finding was not supported by the meta-analysis of data adjusted for important confounders.
- No additional data are available on specific congenital malformations.
Women with asthma are at increased risk of preterm birth and having a low birth weight baby. A systematic review and meta-analysis of preterm birth and birth weight outcomes in women with asthma in pregnancy showed that having an asthma exacerbation in pregnancy increased the odds for having a low birth weight (aOR, 1.15; 95% CI, 1.02–1.29) or small for gestational age (aOR, 1.13; 95% CI, 1.04–1.23) baby, and these data are consistent with previous analyses.8,9 No significant association was found between asthma severity, oral corticosteroid use or exacerbations and preterm birth.8 However, an earlier meta-analysis showed that women with asthma had a 41% increased risk of preterm birth, which was reduced with active asthma management.10
Outcomes in Indigenous Australian populations
Data from over 25,000 Indigenous mothers and babies in Western Australia showed that having asthma was associated with emergency caesarean section (aOR, 1.27; 95% CI, 1.13–1.44), threatened preterm labour and placental abruption, but not with low birth weight or preterm birth.11 Indigenous women who had exacerbations of asthma (defined as hospitalisation or emergency department visit) were at increased odds of threatened preterm labour (aOR, 1.86; 95%, CI 1.37–2.54) and placental abruption (aOR, 2.85; 95%, CI 1.24–6.55) compared with Indigenous women without exacerbations. Among Indigenous women living in more remote areas, asthma was also associated with several adverse pregnancy outcomes, including increased risk of emergency and elective caesarean sections, NICU admission and preeclampsia.11 In a Queensland study, the prevalence of asthma in pregnancy was higher in Indigenous women (24%) compared with non-Indigenous women (17%).2 Compared with non-Indigenous women without asthma, Indigenous women with asthma were more likely to have a neonatal death, preterm birth, low birth weight baby or NICU admission.2 More research is needed to understand community needs and codesign culturally safe models of care for Aboriginal and Torres Strait Islander women with asthma during pregnancy.
Worsening symptoms and exacerbations during pregnancy
One of the unique clinical issues for pregnant women with asthma is the possibility of worsening symptoms due to pregnancy, which are unpredictable and vary between individuals.12 These symptom changes could be related to the immunological changes of pregnancy which favour a T-helper 2 immune environment, mechanical changes related to the growing uterus, or behavioural changes related to medication use and lifestyle factors. The accepted dogma of asthma in pregnancy has been the ‘one-third rule’, where one-third of patients worsen, one-third improve and one third show no change in symptoms.14 However, a recent study from the US of more than 300 women with asthma showed a pattern of worsening symptoms in 40%, whereas 60% showed no change during pregnancy.14 Of interest is the apparent improvement in symptoms seen among all women in the last few weeks of pregnancy. Understanding the underlying mechanism of this pattern could help in the development of new therapeutic targets or approaches for preventing worsening asthma in pregnancy.
Exacerbations occur in around 20% of women with asthma during pregnancy.15-17 A systematic review and meta-analysis identified moderate or severe asthma (compared with having mild asthma) as the biggest risk factor for exacerbations in pregnancy, with a greater than threefold increased risk (RR, 3.44; 95% CI, 2.03–5.83).18 Other key risk factors include having multiple pregnancies (RR, 2.23; 95% CI, 1.10–4.50), depression or anxiety (RR, 1.42; 95% CI, 1.27–1.59), maternal smoking (RR, 1.35; 95% CI, 1.04–1.75), multiparity (RR, 1.31; 95% CI, 1.01–1.68) and maternal obesity (RR, 1.25; 95% CI, 1.15–1.37). An increased awareness of the exacerbation risks associated with nonmodifiable factors, such as multiparity and multiple pregnancy, may lead to proactive management. Targeting modifiable risk factors, such as smoking and depression or anxiety, may also help improve asthma outcomes in women. Smoking and depression or anxiety during pregnancy are associated with the development of asthma or worse asthma control in offspring, making them important targets for management during pregnancy where possible.19,20
Asthma self-management skills in pregnancy
Although the use of ICS reduces the risk of asthma attacks, asthma self-management skills are poor among pregnant women.17,21 GPs have an opportunity to assess and improve these skills. Baseline data from 1200 pregnant women with asthma showed that fewer than 15% had a written asthma action plan, fewer than 30% understood what their medications were for, and fewer than 50% had adequate or optimal pressurised metered dose inhaler (pMDI) technique.17 Only 42% of women used ICS at the first study visit, which is similar to the prevalence of ICS reported globally.22 Of those using ICS for maintenance therapy, 78% were prescribed ICS/long-acting beta agonist (LABA) combination therapy; however, more than one-third were poorly adherent to the prescribed dose and self-reported missing at least 20% of their doses in the past week. Stopping prescribed asthma medications during pregnancy increases the risk of asthma attacks.3
An Australian study of more than 1600 pregnant women with asthma identified risk factors for poor adherence to ICS in early- mid pregnancy (n=610) and persistent poor adherence to ICS during pregnancy (n=612).23 Risk factors for poor adherence included being a current or ex-smoker, being of non-Caucasian or Indigenous Australian descent, having had an adult diagnosis of asthma and having lower lung function. Risk factors for persistent poor adherence throughout pregnancy were associated with lower maternal age, higher parity and not being prescribed ICS in early pregnancy.23 This study describes important characteristics of patients who might require more education around adherence to ICS in pregnancy.
Another study asked participants why they did not like taking asthma medications in pregnancy.24 The most common reason given was the unpleasant taste or throat or mouth symptoms, which can be alleviated by mouth rinsing after use and using a spacer for pMDIs. The second most common reason, cited by one in five women, was feeling that their medications might be unsafe in pregnancy.24 Educating pregnant women about the correct use of asthma medications, their safety and maintaining good asthma control during pregnancy is a key way that all health professionals, including GPs, can improve outcomes for women with asthma during pregnancy.
Lung function and airway inflammation tests
Spirometry
Spirometry is a test for assessing lung function that can be used in primary care, and is generally safe to perform during most of pregnancy (with the exception of late-term pregnancy), provided the woman has no contraindications related to increased myocardial demand or blood pressure, intracranial or intraocular pressure, sinus or middle ear pressure, intrathoracic or intra-abdominal pressure, or transmissible infections.25 A recent study examined changes in forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) during pregnancy among 770 pregnant women with asthma and 259 pregnant women without asthma.26 Although having asthma decreased overall lung function, women with predominantly mild asthma had minimal changes in FEV1 and FVC throughout pregnancy. These results indicate that spirometry is still a clinically useful tool in pregnancy and that any changes in lung function of a pregnant patient with asthma are likely to be because of asthma rather than the pregnancy itself.
Fractional exhaled nitric oxide
We have previously summarised the existing knowledge about using fractional exhaled nitric oxide (FeNO) levels to guide ICS treatment in pregnancy.27 This approach uses an algorithm to increase the ICS dose when FeNO levels are high, decrease the ICS dose when FeNO levels are low and make no changes to the dose when FeNO levels are mid-range. A LABA is added when symptoms remain uncontrolled. The efficacy study using this approach, published in 2011, showed a 50% reduction in exacerbations during pregnancy.28 However, the more recently published Breathing for Life Trial (BLT) showed no significant differences in primary outcome (a composite of adverse perinatal outcomes: preterm birth, small for gestational age, neonatal hospitalisation or perinatal mortality) or exacerbations of asthma in pregnancy between FeNO-based management and usual care among 1200 pregnant women with asthma (including smokers and non-smokers) from six antenatal clinic sites around Eastern Australia.17 The FeNO-based treatment algorithm will require more optimisation if this approach is to be pursued for use in pregnancy. In addition, FeNO is not currently routinely available to GPs.
Treatable traits for asthma in pregnancy
A treatable traits approach that uses a personalised medicine strategy has been proposed to manage many airway diseases, including asthma in pregnancy.29 This approach has been used to identify treatable traits in people with severe asthma, with promising results.30 Traits must be clinically relevant to asthma, measurable and treatable, and fall within three domains:
- pulmonary traits (e.g. airway inflammation)
- extrapulmonary traits (e.g. rhinitis, gastroesophageal reflux, depression and anxiety)
- behavioural traits (e.g. smoking, poor inhaler technique, poor diet, physical inactivity).
The BLT study showed a decrease in FeNO levels and an improvement in asthma control in the intervention group, indicating that the intervention was favourably impacting the traits being targeted (eosinophilic airway inflammation and symptoms causing airflow obstruction).17 A broader treatable traits approach may be useful in the context of pregnancy, and future research should focus on traits with unknown prevalence and clinical relevance in the context of pregnancy. The ideal model of care would target traits that are strongly associated with exacerbations in pregnancy, important for maternal and offspring health, and aligned with the clinical goals of the woman and her managing clinician.
Resources for health professionals, pregnant women with asthma and their family
Most pregnant women with asthma have mild disease and do not require referral to a specialist; therefore, a large proportion of pregnant women will have their asthma solely managed by their GP. In addition, many Australian women have shared obstetric care for their pregnancies. A recent survey showed that midwives and obstetricians lacked the knowledge and confidence to manage asthma in pregnancy, and many did not access guidelines specific to asthma in pregnancy.31 However, these health professionals have regular interactions with pregnant women, giving them the opportunity to contribute to optimising asthma management in pregnancy, and have indicated that they would like to do so.32 Involving obstetricians and midwives in the care of pregnant women requires improved communication between health providers. Antenatal clinicians should reinforce safety messages about asthma medication use in pregnancy and encourage patients to have and follow a written asthma action plan. Women with asthma in pregnancy are at increased risk of infection with respiratory viruses and poor outcomes as a result of infection.33-35 Therefore, clinicians also have an important role in educating pregnant patients on the importance and safety of vaccination during pregnancy and encouraging immunisation, particularly against influenza and SARS-CoV-2 infections.
Our team developed the Asthma in Pregnancy Toolkit, a world-first online resource for health professionals and families (https://asthmapregnancytoolkit.org.au). The Toolkit website contains six information modules for health professionals (Asthma in pregnancy overview, Medications, Treatable traits, Maternity care, Air quality and COVID-19), as well as information for families. The Toolkit and other useful resources for asthma management in pregnancy are summarised in Box 1.
Postpartum care
Regardless of shared care arrangements in pregnancy, it is crucial that a woman with asthma returns to her GP for asthma care after birth. For some women, the first postpartum year is another time of change in asthma symptoms and increased exacerbation risk, possibly because of hormonal changes. Although research in this area is limited, an older study showed that symptoms can worsen in the first three months postpartum in over 50% of women, particularly in those who had an improvement during pregnancy.14 Self-reported ICS non-adherence was higher at six months postpartum than at any time in pregnancy.21 Most asthma medications are safe to use while breastfeeding, and breastfeeding should be encouraged among women with asthma for many reasons, including the potential for reduced wheeze and bronchiolitis in infants.36,37 Despite these potential health benefits for offspring, women with asthma are less likely to initiate breastfeeding (88% vs 98% in the general population) or to be breastfeeding at six months (38% vs 68% in the general population).36,38 New strategies are needed to improve breastfeeding among women with asthma.
Conclusion
Asthma is common and exacerbations during pregnancy can have adverse outcomes for mother and baby. Most SABAs, ICS, ICS/LABA and oral corticosteroids are safe to continue in pregnancy and lactation, where indicated for asthma. Women with asthma should be educated on the importance of continued use of their prescribed asthma medication if they become pregnant. Self-management skills, such as proper inhaler technique and medication adherence, should be optimised, and a written asthma action plan provided to every woman with asthma. Targeting management of modifiable risk factors and an awareness of nonmodifiable risk factors that contribute to exacerbations can help improve asthma outcomes in pregnancy. Practice points for GPs on managing asthma in pregnancy are listed in Box 2. RMT
COMPETING INTERESTS: Associate Professor Murphy is a Project Leader and part of the Core Writing Group for the Asthma in Pregnancy Toolkit. Dr Jensen is a contributor to the Asthma in Pregnancy Toolkit.
References
1. Robinson JL, Gatford KL, Hurst CP, Clifton VL, Morrison JL, Stark MJ. Do improvements in clinical practice guidelines alter pregnancy outcomes in asthmatic women? A single-center retrospective cohort study. J Asthma 2023; 60: 1907-1917.
2. Clifton VL, Das J, Flenady V, Rae K. Neonatal death is a major concern for Indigenous women with asthma during pregnancy and could be prevented with better models of care. Aust N Z J Obstet Gynaecol 2022; 62: 160-163.
3. McLaughlin K, Foureur M, Jensen ME, Murphy VE. Review and appraisal of guidelines for the management of asthma during pregnancy. Women Birth 2018; 31: e349-e57.
4. Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020; 55: 1901208.
5. Namazy JA, Blais L, Andrews EB, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol 2020; 145: 528-536.
6. Robijn AL, Harvey SM, Jensen ME, et al. Adverse neonatal outcomes in pregnant women with asthma: an updated systematic review and meta-analysis. Int J Gynecol Obstet 2024 Feb 7. Epub ahead of print.
7. Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120: 812-822.
8. Xu Z, Doust JA, Wilson LF, Dobson AJ, Dharmage SC, Mishra GD. Asthma severity and impact on perinatal outcomes: an updated systematic review and meta-analysis. BJOG 2022; 129: 367-377.
9. Namazy JA, Murphy VE, Powell H, Gibson PG, Chambers C, Schatz M. Effects of asthma severity, exacerbations and oral corticosteroids on perinatal outcomes. Eur Respir J 2013; 41: 1082-1090.
10. Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118: 1314-1323.
11. Brew BK, Gibberd A, Marks GB, et al. Maternal asthma in Australian indigenous women and perinatal outcomes: a whole population-linked study. Int J Gynecol Obstet 2023; 160: 653-660.
12. Kircher S, Schatz M, Long L. Variables affecting asthma course during pregnancy. Ann Allergy Asthma Immunol 2002; 89: 463-466.
13. Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988; 81: 509-517.
14. Stevens DR, Perkins N, Chen Z, et al. Determining the clinical course of asthma in pregnancy. J Allergy Clin Immunol Pract 2022; 10: 793-802.
15. Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol 2003; 112: 283-288.
16. Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol 2005; 106: 1046-1054.
17. Murphy VE, Jensen ME, Holliday EG, et al. Effect of asthma management with exhaled nitric oxide versus usual care on perinatal outcomes. Eur Respir J 2022; 60: 2200298.
18. Robijn AL, Bokern MP, Jensen MJ, Barker D, Baines KJ, Murphy VE. Risk factors for asthma exacerbations during pregnancy: a systematic review and meta-analysis. Eur Respir Rev 2022; 31: 220039.
19. Brew BK, Lundholm C, Viktorin A, Lichtenstein P, Larsson H, Almqvist C. Longitudinal depression or anxiety in mothers and offspring asthma: a Swedish population-based study. Int J Epidemiol 2018; 47: 166-174.
20. Moore LE, Serrano-Lomelin J, Rosychuk RJ, et al. Perinatal and early life factors and asthma control among preschoolers: a population-based retrospective cohort study. BMJ Open Respir Res 2023; 10: e001928.
21. Robijn AL, Jensen ME, Gibson PG, et al. Trends in asthma self-management skills and inhaled corticosteroid use during pregnancy and postpartum from 2004 to 2017. J Asthma 2019; 56: 594-602.
22. Robijn AL, Jensen ME, McLaughlin K, GIbson PG, Murphy VE. Inhaled corticosteroid use during pregnancy among women with asthma: a systematic review and meta-analysis. Clin Exp Allergy 2019; 49: 1403-1417.
23. Robijn AL, Barker D, Gibson PG, et al. Factors associated with nonadherence to inhaled corticosteroids for asthma during pregnancy. J Allergy Clin Immunol Pract 2021; 9: 1242-1252.
24. Murphy VE, Robijn AL, Metcalfe TB, et al. Beliefs about medicines and adherence to asthma medications during pregnancy. J Asthma 2023; 60: 1446-1454.
25. National Asthma Council Australia. The spirometry handbook for primary care. Melbourne: National Asthma Council Australia; 2023. Available online at: https://www.nationalasthma.org.au/living-with-asthma/resources/health-professionals/information-paper/spirometry-handbook (accessed April 2024).
26. Jensen ME, Robijn AL, Gibson PG, Oldmeadow C; Managing Asthma in Pregnancy study collaborative group; Breathing for Life Trial collaborative group; Murphy VE. Longitudinal analysis of lung function in pregnant women with and without asthma. J Allergy Clin Immunol Pract 2021; 9: 1578-1585.
27. Murphy VE, Jensen ME, Gibson PG. Managing asthma in pregnancy. Respiratory Med Today 2018; 3(1): 14-17.
28. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378: 983-990.
29. Joshi E, Gibson PG, McDonald VM, Murphy VE. Treatable traits in asthma during pregnancy: a call for a shift towards a precision-based management approach. Eur Respir Rev 2023; 32: 230105.
30. McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology 2019; 24: 37-47.
31. Wright TK, McLaughlin K, Jensen ME, Robijn AL, Foureur M, Murphy VE. A cross-sectional survey of Australian healthcare professionals’ confidence, evidence-based knowledge and guideline use for antenatal asthma management. Aust N Z J Obstet Gynaecol 2022; 62: 681-687.
32. McLaughlin K, Kable A, Ebert L, Murphy VE. Midwives’ perception of their role in providing antenatal asthma management in Australia - a qualitative study. Midwifery 2016; 35: 11-16.
33. Murphy VE, Powell H, Wark PA, Gibson PG. A prospective study of respiratory viral infection in pregnant women with and without asthma. Chest 2013; 144: 420-427.
34. Aabakke AJM, Peterson TG, Wojdemann K, et al. Risk factors for and pregnancy outcomes after SARS-CoV-2 in pregnancy according to disease severity: a nationwide cohort study with validation of the SARS-CoV-2 diagnosis. Acta Obstet Gynecol Scan 2023; 102: 282-293.
35. Hartert TV, Neuzil KM, Shintani AK, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol 2003; 189: 1705-1712.
36. Harvey SM, Murphy VE, Gibson PG, et al. Maternal asthma, breastfeeding, and respiratory outcomes in the first year of life. Pediatr Pulmonol 2020; 55: 1690-1696.
37. Harvey SM, Murphy VE, Whalen OM, Gibson PG, Jensen ME. Breastfeeding and wheeze-related outcomes in high-risk infants: a systematic review and meta-analysis. Am J Clin Nutr 2021; 113: 1609-1618.
38. Netting MJ, Moumin NA, Knight EJ, Golley RK, Makrides M, Green TJ. The Australian Feeding Infants and Toddler Study (OzFITS 2021): breastfeeding and early feeding practices. Nutrients 2022; 14: 206.