Severe asthma: what’s new in management?

The burden of severe asthma is significant and needs to be better recognised and managed by patients and clinicians. Advances in severe asthma management include machine learning to optimise diagnosis, case finding, smart inhaler technologies and implementation of a structured multidisciplinary approach. Treatment strategies focusing on treatable traits and available biologics should help reduce asthma morbidity, including cumulative harm from the use of oral corticosteroids.
- Severe asthma is under-recognised, often resulting in delayed access to specialist input.
- Case finding through machine learning, integrating clinical decision support tools and improving access to smart inhaler technology are feasible ways to optimise asthma care using available technology.
- A structured multidisciplinary approach improves severe asthma diagnosis and outcomes. Aspects of this approach can be implemented in primary care.
- Asthma outcomes are significantly improved with availability of biologic therapies and adoption of the treatable traits approach.
Asthma is a highly prevalent heterogeneous condition that is both over- and underdiagnosed.1,2 In high-income countries, including Australia, people with severe asthma have disproportionately high healthcare resource utilisation and rates of morbidity and mortality, in addition to significant psychological and socioeconomic burdens.3-6 As such, severe asthma is an important topic for ongoing research and quality improvement initiatives around diagnosis, personalised management and emerging therapeutics.
This article highlights some of the recent advances in the model of clinical care and the promise of new technologies for the management of severe asthma. It also discusses how our increased understanding of asthma pathophysiology is moving us towards more effective treatment and helping to protect patients from accruing lung damage and harm from use of oral corticosteroids (OCS).
Identifying patients with severe asthma
Asthma is diagnosed based on compatible symptoms in the presence of supportive objective information: variable airflow obstruction, airway inflammation or both. Severe asthma identifies the group of patients with persistent uncontrolled asthma, defined by symptom burden, exacerbation or compromised lung function despite optimised inhaled therapy with long-acting beta-agonists and inhaled corticosteroids, and systematic assessment and management of comorbidities.7 About 3 to 10% of people with asthma have severe disease.8 The current paradigm of asthma diagnosis and severity assessment heavily relies on the recognition of clinical features of severe asthma by both patients and clinicians. Previous large-scale, observational studies have found that most patients who meet specialist referral criteria for their asthma remain in primary care or are subjected to prolonged wait times.9,10
Advances in machine learning
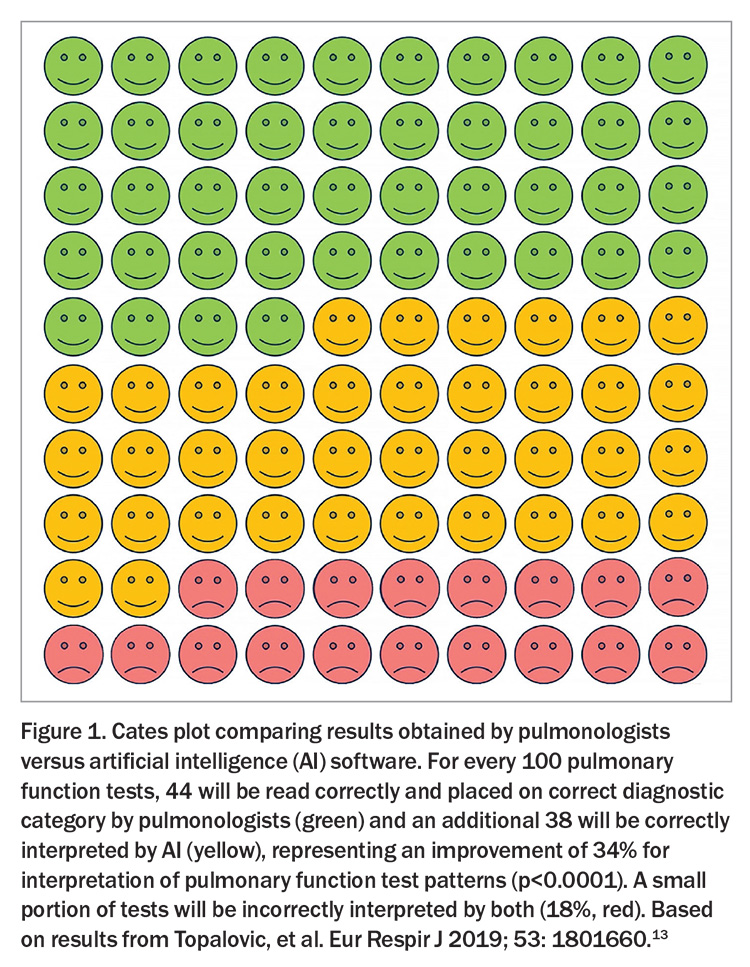
The routine use of electronic medical records in primary care affords an opportunity to identify individuals with uncontrolled asthma earlier, before severe harm has accrued and while there is a higher chance of achieving excellent long-term control. Advances in machine learning capacity and its increased availability have shown great promise in similar scenarios in other disease areas, helping to identify patients with familial hypercholesterolaemia and diabetes and its associated complications through automated analysis of risk factors and test results.11,12 It is already possible to leverage routine clinical data in primary care to flag individuals on the basis of features, such as OCS consumption and persisting eosinophilia, and to highlight comorbidities. With GPs under ever greater time pressure, machine learning-supported approaches to identify patients, report tests (Figure 1) and pre-prepare referrals appears likely to become an essential aspect of primary care.13
Clinical decision support tools
The use of new technologies is also becoming increasingly common as a central aspect of consultation. Clinical decision support tools embedded in electronic health records can help implement guideline-based care in an efficient manner. A recent Norwegian trial investigating the use of web-based, clinical decision support systems in managing chronic obstructive pulmonary disease is one of several that have demonstrated improved diagnostic accuracy with their use. Furthermore, patient management more closely resembled guideline recommendations when the tool was used.14 Both the identification of individuals at risk of poor outcomes and improved quality of supported consultations will be key elements to substantially improve patient care in the near future.
Smart inhaler technologies
Smart inhalers with electronic monitoring devices attached to, or integrated into, the inhaler device provide data on the frequency of device use and adequacy of inhaler technique. They have been used in trials for many years and are now in routine clinical use in other countries. Data from smart inhalers helps identify high salbutamol use, which is an independent risk factor for asthma fatalities, as shown by several studies including the UK National Review of Asthma Deaths. Currently, salbutamol overuse – defined as using greater than three canisters per year – often goes unnoticed,15 particularly in Australia where it is easily accessible over the counter, and escalation of preventive interventions may not be undertaken. Furthermore, smart inhalers can differentiate severe asthma from difficult-to-treat asthma by recording medication concordance for physician review, and have also been shown to promote concordance by providing feedback to patients.16,17 It is worthwhile highlighting that salbutamol monotherapy is associated with increased risk of asthma-related death. Since 2019, international guidelines have resolved that people with asthma should not be treated with salbutamol alone.18
Important features of severe asthma assessment
Using a structured multidisciplinary approach with multiple investigations improves severe asthma diagnosis and outcomes, although complete implementation of this approach is currently limited to a subspecialist care setting.19 However, with improved clinical decision-support tools and case finding capability in primary care, it is important to consider features that form part of specialist assessment and can be recorded or enacted in primary care.
Assessing cumulative OCS toxicity
Cumulative OCS toxicity is resulting in an increasingly frail and multimorbid asthma population. A recent important finding has been the very low threshold at which this toxicity begins – a lifetime cumulative dose of 1 g or less.20 In recent years, a retrospective study using PBS dispensing data estimated that over a quarter of people with asthma have reached this threshold.21 Moreover, the range of organ systems affected by OCS toxicity is larger than originally thought. Despite this increased risk, people with asthma and their healthcare practitioners may not immediately attribute issues, such as a deep vein thrombosis, to OCS use.22 Harm reduction strategies aimed at minimising OCS prescribing have been proposed by the Thoracic Society of Australia and New Zealand.20 This includes addressing issues of appropriate diagnosis, optimisation of inhaler therapy, management of risk factors and comorbidities and prompt referral of at-risk patients for specialist review for consideration of steroid-sparing advance therapies, such as biologics.
Clarifying gestational age
Advances in neonatal critical care means more children born preterm or very preterm are surviving into adulthood.23 Impaired lung function at birth for any reason, including prematurity, often persists into adulthood, with trajectories further affected by other early life and adult risk factors.24 This cohort are therefore more likely to have an asthma diagnosis and be more poorly controlled. Clarifying gestational age in people with asthma is therefore recommended as a way to better understand their lung pathology and to identify individuals at higher risk of dysfunction in other organs such as the heart.
Measuring exhaled nitric oxide levels
For decades we have assessed asthma using a marker of airflow obstruction (spirometry) but have relatively neglected the other key element of the condition: airway inflammation. Measuring exhaled nitric oxide as a simple point of care test for airway inflammation can help with diagnostics and identify people who are likely to respond to inhaled corticosteroids. Fractional exhaled nitric oxide (FeNO) can also help predict response to some biologic therapies, with individuals who have high levels (>40 ppb) being very likely to respond well compared with those who have intermediate (25 to 40 ppb) or normal (<25 ppb) levels.25 A persistently elevated FeNO level despite use of appropriate inhaled corticosteroid and long-acting beta-2 agonist treatment may indicate treatment-resistant disease. However, it more often indicates an issue with preventer adherence, inhaler technique or uncontrolled comorbid respiratory inflammation such as allergic rhinitis.26 FeNO suppression testing with a smart inhaler device preventer could delineate these issues. Although multiple large trials have assessed whether personalised FeNO-guided treatment can aid in exacerbation prevention, symptom control or OCS avoidance, the results have not been consistent in adults or children.27 This may be due to the variations between trial protocols and further studies are needed to clarify the role of FeNO-adjusted management. Nevertheless, FeNO remains a useful point of care test along with phenotyping blood, including eosinophil counts and immunoglobulin E tests.
Emerging treatments for severe asthma
Identifying and treating clinically relevant traits
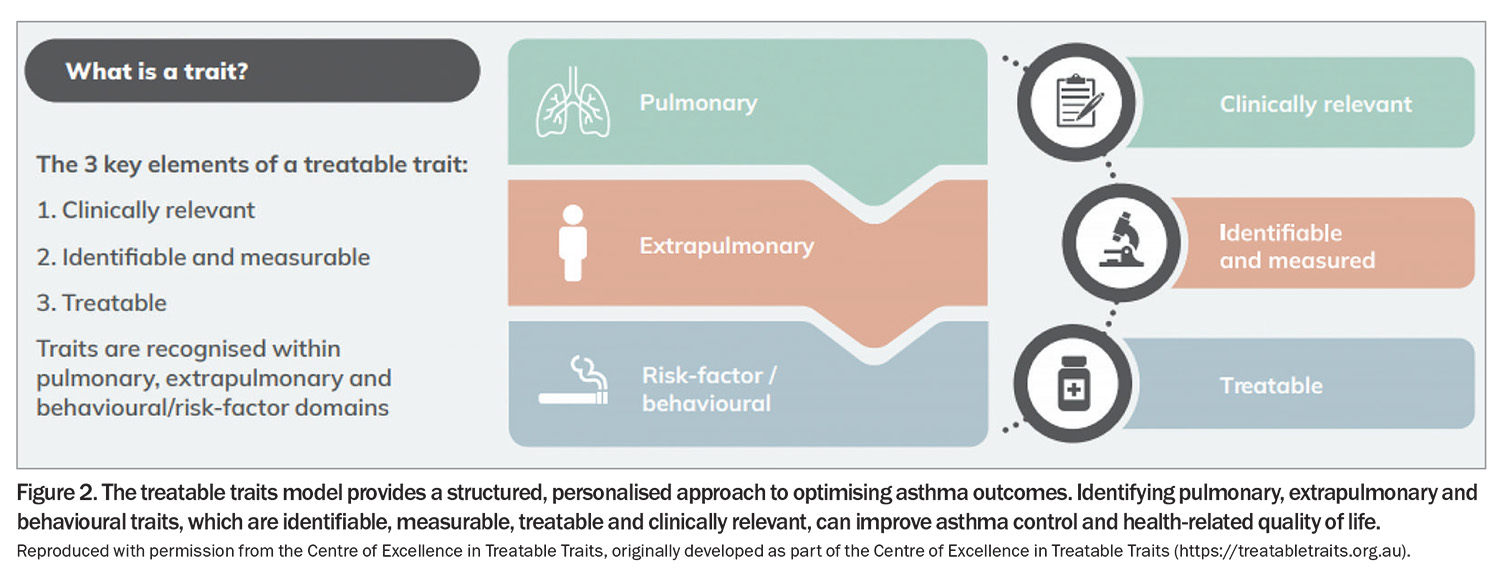
The treatable traits model of care provides an increasingly practical, label-free approach to personalised management for a heterogeneous disease and patient population. Identifying clinically relevant traits that respond to targeted management can optimise outcomes for patients with asthma (Figure 2). This approach has broadened the delivery of asthma care. Although not an exhaustive list, traits such as obesity, anxiety and depression, poor adherence patterns, uncontrolled allergic rhinitis, gastro-oesophageal reflux disease, smoking and limited self-management skills can be addressed and their impacts mitigated.
Diagnosing and managing vocal cord dysfunction
Of increasing interest is the diagnosis and management of inducible laryngeal obstruction (also known as vocal cord dysfunction), which frequently mimics and often coexists with asthma. Advanced testing, such as flexible nasoendoscopy with perfume provocation, dynamic CT of the larynx, or continuous laryngoscopy during exercise, can identify the sometimes subtle, transient laryngeal narrowing on inspiration, which is the hallmark of this treatable condition.28-30 Speech pathology-led multidisciplinary management using techniques, such as biofeedback and laryngeal retraining, can substantially improve the symptom burden of these patients.
Identifying small airways disease
Small airways disease is more prevalent in severe asthma and has important associations with asthma control and exacerbations.31,32 The identification of small airways disease and ventilation heterogeneity is an area of increasing interest. New investigations, such as oscillometry, functional imaging modalities and electrical impedance tomography, are becoming more widely used.33-35 More clinical data on the potential benefits of extra fine particle inhaled therapies in such patients are awaited with interest.
Biologics and emerging drug classes
Monoclonal antibody (biologic) drugs have provided a breakthrough in severe asthma management over the past two decades. They have significantly improved exacerbation rates and asthma control and reduced OCS burden for individuals with type 2 inflammatory profiles. The impressive efficacy of biologics in well phenotyped patients has prompted analyses of ‘super-responders’ and reinvigorated discussion around the possibility of asthma remission. This raised expectations of what is possible in asthma is most welcome, and inducing remission in asthma is likely to prevent the commonly seen physical and psychological effects of acute asthma and its treatment. However, there is not yet a consensus on what defines clinical remission or complete biological remission on and off treatment, and how this label affects management and future treatment considerations.36,37 To achieve the goal of remission for all, therapies that target other pathways in asthma and that are more affordable at scale are needed. These must include targeting airway smooth muscle and remodelling, an aspect of asthma relatively lacking in effective treatments, and also the recent elucidated and biologically important airway adipose tissue.
Beyond biologics, newer drugs in other classes are under investigation in asthma, such as small interfering RNA (siRNA) and inhaled anticalins, giving hope for an inhaled biologic in the near future.38-40 Reinvestigating and repurposing existing medications from other disease areas may also be a fruitful avenue, such as dexpramipexole for eosinophilic asthma,41 and azithromycin to influence efferocytosis and reduce airway inflammation. The potential for biologics to reduce future OCS use for acute exacerbations is well established, but the direct replacement of rescue courses of steroids with a dose of a monoclonal antibody is an exciting area of research. A randomised controlled trial of acute benralizumab use has recently completed recruitment.
Pulmonary rehabilitation
Although the progress in asthma pharmacotherapies grabs headlines, the huge potential benefit of nonpharmacological interventions cannot be ignored. Pulmonary rehabilitation has repeatedly demonstrated its indispensable place in chronic obstructive pulmonary disease management; however, its role in asthma is less well defined. A recently published systematic review and meta-analysis explored the beneficial effects of pulmonary rehabilitation on quality of life, exercise tolerance and lung function in patients with asthma, and called for large-scale quality randomised control trials to further investigate promising signals.42
An international severe asthma registry
A welcome development in recent years has been the increase in the number of national registries of individuals with severe asthma, and their incorporation into an international registry (www.isar.opcglobal.org). The findings from such platforms have already influenced the international asthma guidelines and continue to produce fruitful collaborative research.
Conclusion
Severe asthma is a rapidly evolving area of medicine. Case finding through machine learning, integrating clinical decision support tools and improving access to smart inhaler technology are feasible ways to optimise asthma care using available technology. OCS harm minimisation strategies are under investigation following the recent finding of the low cumulative dose threshold for toxicity. Furthermore, implementing a practical model of care that encourages personalised and holistic asthma management can improve asthma care while broadening research opportunities for otherwise overlooked conditions. Finally, the emergence of novel biomarkers and drug classes further builds on the momentum and enthusiasm generated in the biologic era, potentially ushering in a new era of asthma remission built on international collaborative research. RMT
COMPETING INTERESTS: Dr Crawford has received speaker fees from Chiesi.
Dr Blakey has received medical advisory contract fees from Asthma Australia; research grants from MRFF, FHRI, Telethon Kids Institute, International Primary Care Respiratory Group, Charlies Foundation for Research; personal and/or institutional fees for educational presentations from Chiesi, The Limbic, Boehringer Ingelheim, GSK, AstraZeneca and Sanofi; and support for travel to conferences from GSK, Centre for Research Execellence in Treatable Traits, AstraZeneca and The George Institute. Dr Chung has received speaker fees from Chiesi, GSK, AstraZeneca and Boehringer Ingelheim.
References
1. Adams RJ, Wilson DH, Appleton S, et al. Underdiagnosed asthma in South Australia. Thorax 2003; 58: 846-850.
2. Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 2018; 198: 1012-1020.
3. Roche N, Garcia G, de Larrard A, et al. Real-life impact of uncontrolled severe asthma on mortality and healthcare use in adolescents and adults: findings from the retrospective, observational RESONANCE study in France. BMJ Open 2022; 12: e060160.
4. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest 2020; 157: 790-804.
5. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765.
6. Gupta RP, Mukherjee M, Sheikh A, Strachan DP. Persistent variations in national asthma mortality, hospital admissions and prevalence by socioeconomic status and region in England. Thorax 2018; 73: 706-712.
7. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2023. Available online at: https://ginasthma.org/2023-gina-main-report/ (accessed April 2024).
8. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343-373.
9. Blakey JD, Gayle A, Slater MG, Jones GH, Baldwin M. Observational cohort study to investigate the unmet need and time waiting for referral for specialist opinion in adult asthma in England (UNTWIST asthma). BMJ Open 2019; 9: e031740.
10. Ryan D, Heatley H, Heaney LG, et al. Potential severe asthma hidden in UK Primary Care. J Allergy Clin Immunol Pract 2021; 9: 1612-1623.
11. Luo RF, Wang JH, Hu LJ, Fu QA, Zhang SY, Jiang L. Applications of machine learning in familial hypercholesterolemia. Front Cardiovasc Med 2023; 10: 1237258.
12. Zhang Z, Yang L, Han W, et al. Machine learning prediction models for gestational diabetes mellitus: meta-analysis. J Med Internet Res 2022; 24: e26634.
13. Topalovic M, Das N, Burgel PR, et al. Artificial intelligence outperforms pulmonologists in the interpretation of pulmonary function tests. Eur Respir J 2019; 53: 1801660.
14. Vijayakumar VK, Mustafa T, Nore BK, et al. Role of a digital clinical decision-support system in general practitioners’ management of COPD in Norway. Int J Chron Obstruct Pulmon Dis 2021; 16: 2327-2336.
15. Janson C, Menzies-Gow A, Nan C, et al. SABINA: an overview of short-acting
β(2)-agonist use in asthma in european countries. Adv Ther 2020; 37: 1124-1135.
16. Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol 2007; 119: 811-816.
17. Chan AH, Stewart AW, Harrison J, Camargo CA Jr, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med 2015; 3: 210-219.
18. Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J 2019; 53: 1901046.
19. Clark VL, Gibson PG, Genn G, Hiles SA, Pavord ID, McDonald VM. Multidimensional assessment of severe asthma: a systematic review and meta-analysis. Respirology 2017; 22: 1262-1275.
20. Blakey J, Chung LP, McDonald VM, et al. Oral corticosteroids stewardship for asthma in adults and adolescents: a position paper from the Thoracic Society of Australia and New Zealand. Respirology 2021; 26: 1112-1130.
21. Hew M, McDonald VM, Bardin PG, et al. Cumulative dispensing of high oral corticosteroid doses for treating asthma in Australia. Med J Aust 2020; 213: 316-320.
22. Waljee, AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017; 357: j1415.
23. Evans DJ, Gibbons JTD, Simpson S.J. Blakey JD. Preterm lung disease: not just for neonatologists. Respir Med Today 2023; 8(2): 21-24.
24. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018; 6: 535-544.
25. Price DB, Buhl R, Chan A, et al. Fractional exhaled nitric oxide as a predictor of response to inhaled corticosteroids in patients with non-specific respiratory symptoms and insignificant bronchodilator reversibility: a randomised controlled trial. Lancet Respir Med 2018; 6: 29-39.
26. d’Ancona G, Kent BD. Practical applications of FeNO measurement and inhaler monitoring technologies in the management of difficult asthma. J Allergy Clin Immunol Pract 2021; 9: 1539-1540.
27. Korevaar DA, Damen JA, Heus P, et al. Effectiveness of FeNO-guided treatment in adult asthma patients: A systematic review and meta-analysis. Clin Exp Allergy 2023; 53: 798-808.
28. Clemm HH, Olin JT, McIntosh C, et al. Exercise-induced laryngeal obstruction (EILO) in athletes: a narrative review by a subgroup of the IOC Consensus on ‘acute respiratory illness in the athlete’. Br J Sports Med 2022; 56: 622-629.
29. Rogde ÅJ, Lehmann S, Halvorsen T, et al. Prevalence and impact of exercise-induced laryngeal obstruction in asthma: a study protocol for a cross-sectional and longitudinal study. BMJ Open 2023; 13: e071159.
30. Koh J, Phyland D, Baxter M, Leong P, Bardin PG. Vocal cord dysfunction/inducible laryngeal obstruction: novel diagnostics and therapeutics. Expert Rev Respir Med 2023; 17: 429-445.
31. Agusti A, Gibson PG, McDonald VM, Treatable traits in airway disease: from theory to practice. J Allergy Clin Immunol Pract 2023; 11: 713-723.
32. van der Wiel E, ten Hacken NH, Postma DS, van den Berge M. Small-airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J Allergy Clin Immunol. 2013; 131: 646-657.
33. Hsu HJ, Chang HT, Zhao Z, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve: a randomized trial in moderate to severe ARDS. Physiol Meas 2021; 42: 014002.
34. Rutting S, Chapman DG, Farah CS, Thamrin C. Lung heterogeneity as a predictor for disease severity and response to therapy. Curr Opin Physiol 2021; 22: 100446.
35. Longhini F, Bruni A, Garofalo E. Chest physiotherapy improves lung aeration in hypersecretive critically ill patients: a pilot randomized physiological study. Crit Care 2020; 24: 479.
36. Shah PA, Brightling C. Biologics for severe asthma-which, when and why? Respirology 2023; 28: 709-721.
37. Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission: what is it and how can it be achieved? Eur Respir J 2022; 60: 2102583.
38. Soccio P, Moriondo G, Lacedonia D, et al. MiRNA and exosomal miRNA as new biomarkers useful to phenotyping severe asthma. Biomolecules 2023; 13: 1542.
39. Matschiner G, Fitzgerald MF, Moebius U, et al. Elarekibep (PRS-060/AZD1402), a new class of inhaled Anticalin medicine targeting IL-4Ra for type 2 endotype asthma. J Allergy Clin Immuno 2023; 151: 966-975.
40. Morales-Kastresana A, Siegemund M, Haak S, Peper-Gabriel J, Neiens V, Rothe C. Anticalin®-based therapeutics: expanding new frontiers in drug development. Int Rev Cell Mol Biol 2022; 369: 89-106.
41. Cusack, RP, Sulaiman I, Gauvreau GM. Refashioning dexpramipexole: a new horizon in eosinophilic asthma? J Allergy Clin Immunol 2023; 152: 1092-1094.
42. Feng Z, Wang J, Xie Y, Li J. Effects of exercise-based pulmonary rehabilitation on adults with asthma: a systematic review and meta-analysis. Respir Res 2021; 22: 33.