Pertussis: the post-COVID-19 resurgence in Australia

Despite high vaccination coverage, pertussis remains endemic in Australia, with cyclical outbreaks driven by waning immunity and vaccine escape variants. The post-COVID-19 resurgence highlights the need for enhanced surveillance, improved vaccination strategies and strengthened clinical awareness to mitigate transmission and protect vulnerable populations.
- Despite high vaccine coverage, pertussis remains endemic, with cyclical outbreaks every three to five years, exacerbated by waning immunity and vaccine escape variants.
- Immunity following either natural infection or vaccination declines over time, necessitating booster doses to maintain protection.
- The rise of pertactin-deficient Bordetella pertussis strains and mutations in pertussis toxin promoter ptxP3 variant may contribute to increased disease severity and transmission.
- Although infants remain the most vulnerable, adolescents and adults with waning immunity now serve as major reservoirs of transmission, often presenting with mild or atypical symptoms.
- Immunisation during pregnancy remains a key strategy to protect infants in the first months of life, yet recent declines in uptake highlight the need for targeted public health messaging.
- Improved genomic monitoring, enhanced diagnostic awareness among GPs, addressing vaccine hesitancy, advocating for household and caregiver ‘cocoon’ vaccinations and expanding vaccine funding will be crucial to mitigating future pertussis outbreaks.
Pertussis, or whooping cough, remains a significant public health concern despite widespread vaccination. Caused primarily by the bacterium Bordetella pertussis, this highly infectious acute respiratory disease has a unique clinical presentation characterised by severe paroxysmal coughing and a prolonged course of illness. It poses a particularly severe threat to incompletely vaccinated infants (who have received fewer than three doses), in whom complications can lead to hospitalisation or death.
Australia has achieved high vaccine coverage through public health measures, including the National Immunisation Program (NIP), contributing to a marked reduction in pertussis incidence and mortality over decades. However, the resurgence of cases following the coronavirus disease 2019 (COVID-19) pandemic raises important questions about waning immunity, vaccine effectiveness and shifting epidemiology. The stark decline in pertussis notifications observed during the pandemic because of public health actions (e.g. mask wearing and physical distancing) has been followed by a profound rebound in cases as these measures have eased.
This resurgence underscores the need for renewed attention among healthcare professionals to the clinical features, diagnosis, management and prevention of pertussis. As frontline clinicians, GPs play a pivotal role in recognising and managing pertussis cases, particularly given the atypical presentations in adolescents and adults, who are often sources of infection for infants.
This article provides a review of pertussis with a focus on the Australian context and recent epidemiological trends. Key topics include the microbiology, pathogenesis, clinical manifestations and diagnosis of pertussis, along with therapeutic approaches and preventive strategies including vaccination. The objective is to equip GPs and other healthcare professionals with the knowledge needed to manage pertussis effectively in this evolving landscape.
Microbiology of Bordetella pertussis and related species
First isolated in 1906 by Bordet and Gengou, B. pertussis is a fastidious, motile Gram-negative, pleomorphic aerobic coccobacillus that exclusively infects humans (although it can be experimentally infected in baboons and mice).1,2 It is the primary causative agent of pertussis and is highly specialised for colonising the ciliated epithelium of the respiratory tract.3,4 Of the 16 described Bordetella species, a further three are considered medically important – Bordetella parapertussis, Bordetella bronchiseptica and Bordetella holmesii – each with similar signs and symptoms.
- B. pertussis is the quintessential aetiological agent causing the most severe forms of whooping cough. Humans are the only known reservoirs.5
- B. parapertussis can cause the classic whooping cough-like illness, although it is considered less severe than infection caused by B. pertussis. Its ability to circulate within populations and cause sporadic outbreaks has been observed, with children often being the most affected.6-8
- B. bronchiseptica infection produces respiratory illness in a wide range of mammals, including dogs (kennel cough). Most human cases are seen in immunocompromised hosts.9-11
- B. holmesii has also been associated with infections in immunocompromised individuals; however, recent Australian data suggest it may be more widespread, cocirculating with B. pertussis and accounting for up to 16.8% of pertussis cases.12
- There are many other Bordetella species and some isolates await further speciation and description.13
Codetection with other respiratory pathogens (e.g. respiratory syncytial virus, adenovirus, human rhinovirus) does occur.14,15
Genetic and phenotypic features
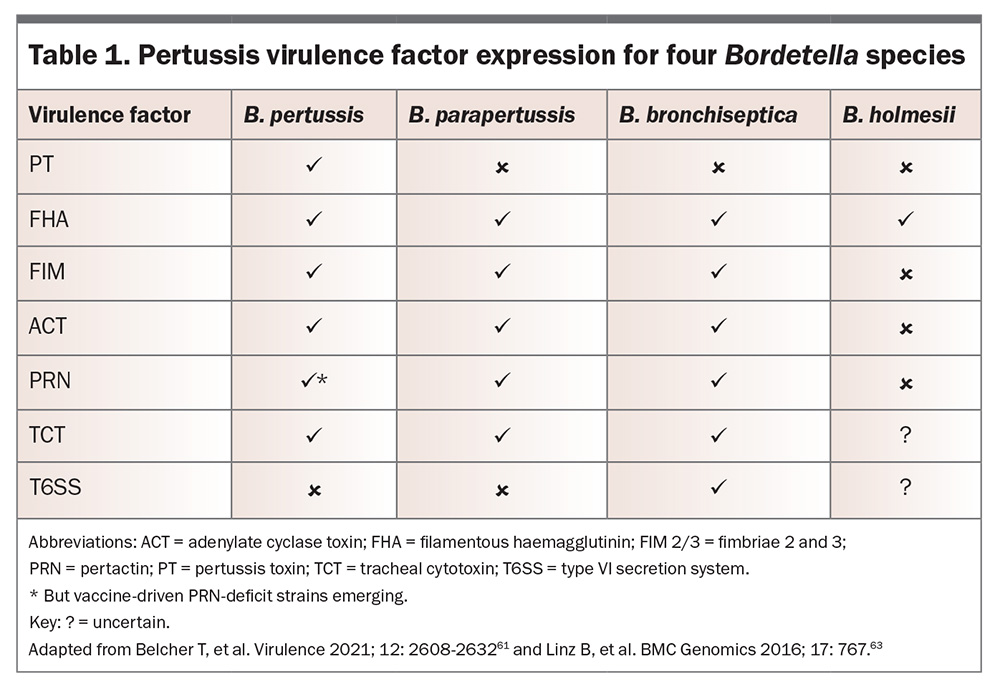
The four medically important Bordetella species (B. pertussis, B. parapertussis, B. bronchiseptica and B. holmesii) exhibit both genetic similarities and significant differences in pathogenicity, host adaptation, virulence factor expression and epidemiological impact. Although all four species can cause respiratory infections, their distinct genomic structures and phenotypic traits shape their disease presentation and public health significance.16 For example, only B. pertussis expresses pertussis toxin (PT), with the other species causing a milder disease course in most cases.17
The genome of B. pertussis is smaller compared with other Bordetella species, with evidence of significant gene loss. These adaptations reflect its specialisation for human hosts.18,19 The bacterium’s strict requirements for growth in vitro, requiring protective substances such as charcoal or blood in culture, are another testament to its niche specificity.20
B. pertussis is distinguished from other Bordetella species by specific genetic elements, such as insertion sequences, which are used as targets in diagnostic nucleic acid amplification testing, most commonly polymerase chain reaction (PCR) assays.21,22
Epidemiology
Pertussis remains a global health concern, with cases reported across all continents. Although vaccination programs have significantly reduced the disease burden, pertussis continues to cause considerable morbidity and mortality. The historically cited basic reproduction number (R₀; the average expected number of infections caused by one case in a susceptible population) for pertussis of 12 to 17, derived from epidemiological data collected between 1908 and 1917 in the United States and 1944 to 1979 in England and Wales, may not accurately reflect current transmission dynamics because of changes in population structure, vaccination coverage and social behaviours.23,24 More recent analyses indicate that R₀ values for pertussis may be lower, with estimates ranging from 5.5 in Europe to between 9 and 12 in the United States and 10 in Australia.25-27
Australian context
Australia has achieved commendable pertussis vaccination coverage in infant, child and adolescent target groups through the NIP. Despite this, pertussis remains endemic throughout Australia, with periodic outbreaks occurring every three to five years.28,29
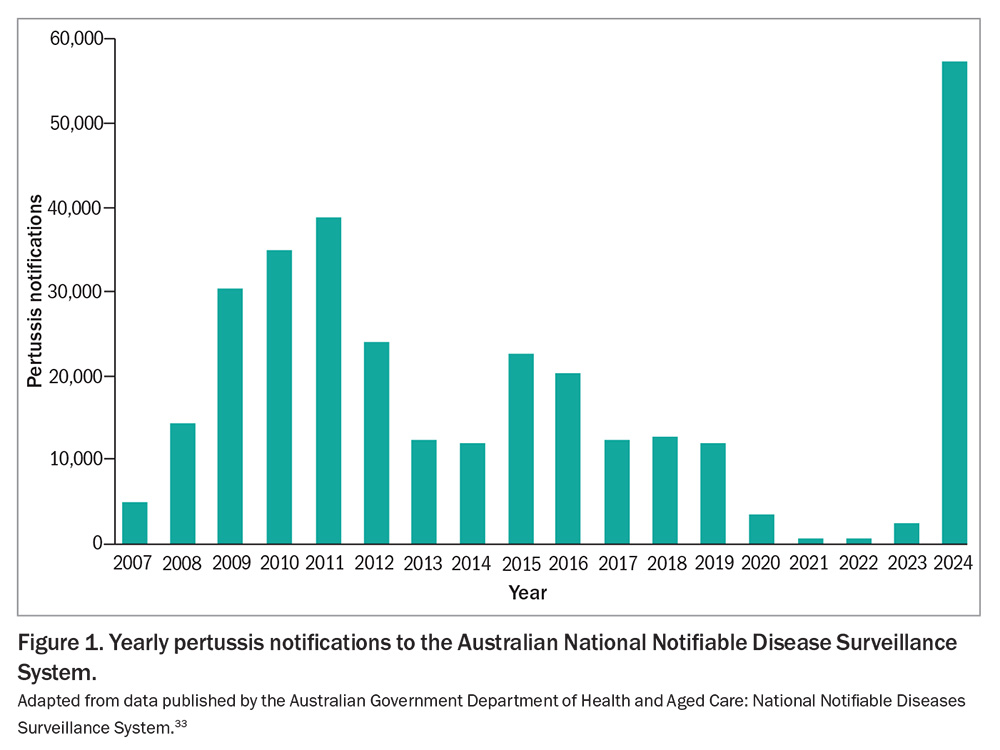
The COVID-19 pandemic had a profound impact on pertussis epidemiology (Figure 1). Public health measures such as physical distancing, mask use and reduced international travel led to an unprecedented decline in reported pertussis cases in Australia and globally.30 However, with the relaxation of these measures, a resurgence of pertussis has been observed.31,32 In 2024, Australia recorded 57,146 pertussis notifications to the National Notifiable Disease Surveillance System, more than 47% higher than the previous peak of 38,748 in 2011.33 For the first three months of 2025 there were 9577 notifications, more than three-fold higher compared with January to March 2024.
Age and risk group dynamics
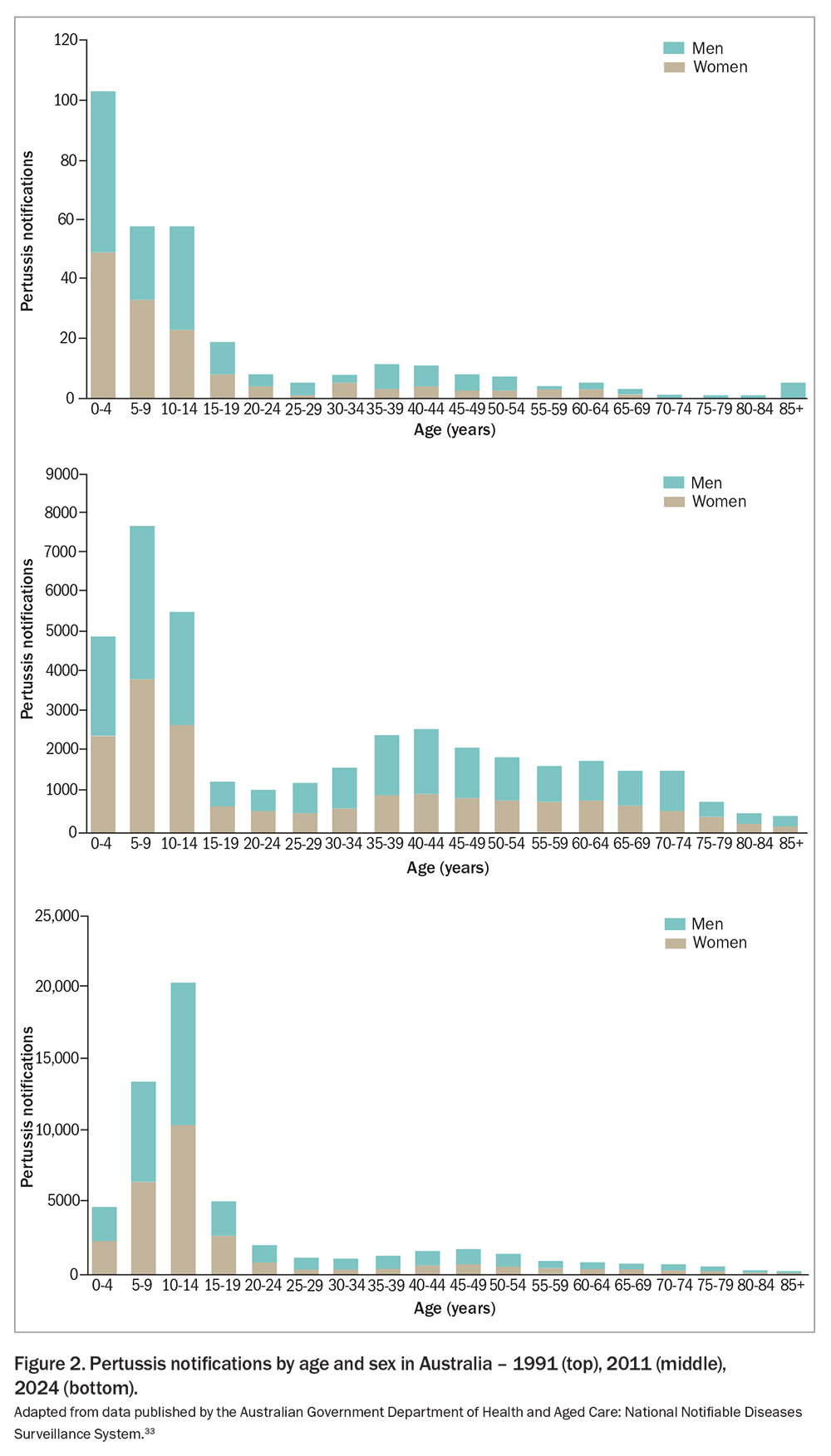
In Australia, the age distribution of pertussis cases has shifted over time (Figure 2). Historically, the incidence of pertussis was the highest in infants and children younger than 5 years of age but, since the late 1990s, this has shifted toward adolescents. However, infants lacking full immunisation remain at the highest risk for severe disease and complications, including hospitalisation and mortality.2,34-36 Data from 2013–18 confirm this, showing infants younger than 2 months of age had the highest rates of pertussis-related hospitalisations (143.2 per 100,000), closely followed by those aged 2 to 3 months (137.8 per 100,000).37 During this period, nine deaths had a causal link with pertussis, of which six were in infants younger than 1 year of age. Siblings, adolescents and adults often serve as reservoirs of infection because of waning immunity, and often present with atypical or mild symptoms.38,39 These groups play a crucial role in transmitting the disease to vulnerable infants.40
Transmission and cyclical patterns
Despite recent advances in animal modelling, much uncertainty remains regarding the mechanism of pertussis transmission.41-44 Pertussis is thought to be transmitted from an infected patient through aerosolised droplets by direct inhalation and via indirect self-inoculation from surrounding contaminated surfaces.41,45 Transmission rates of more than 80% have been observed among susceptible household contacts.46 Bacterium shedding via respiratory secretions is most infectious during the early symptomatic phase, whereas transmission is most efficient once coughing has developed.47 The cyclical nature of pertussis outbreaks, with inter-epidemic periods of three to five years, persists despite widespread vaccination.28,29,44,48,49
Waning immunity and vaccine escape variants
One of the major challenges in pertussis control is waning immunity following either natural infection or vaccination, with ongoing debate regarding the underlying causes and varying impact seen in infants, children, adolescents and adults.44 Although B. pertussis infection induces a robust T helper (Th)1/Th17 immune response, evidence suggests that infection-derived immunity wanes within four to 20 years, whereas vaccine-induced immunity – particularly from acellular pertussis (aP) vaccines – wanes within four to 12 years, necessitating booster doses to maintain protection.42,50,51 Studies indicate that these aP vaccines induce a primarily Th2-skewed response, which fails to generate strong mucosal immunity, leading to asymptomatic colonisation and continued bacterial transmission despite vaccination.52 Although whole-cell inactivated pertussis (wP) vaccines generate a more durable Th1/Th17 response, their high reactogenicity – local reactions such as injection site pain, swelling and redness through to systemic reactions including fever, persistent crying and convulsions – led to widespread disapproval and subsequent replacement with aP vaccines in most high-income countries including Australia from the mid-1990s.
The genomic evolution of B. pertussis has resulted in antigenic divergence from vaccine strains, raising concerns about vaccine-driven selection pressure. Key studies have identified an increase in pertactin (PRN)-deficient visolates, which have been increasingly reported in Australia and other countries using aP vaccines, potentially reducing their effectiveness.27 The loss of PRN, an autotransporter protein, is hypothesised to confer a selective advantage in aP-immunised populations, allowing PRN-deficient strains to persist and spread. Furthermore, mutations in the pertussis toxin promoter ptxP3 variant have been associated with higher toxin production, potentially contributing to increased disease severity.53,54
Demographic disparities
Indigenous Australians face unique challenges regarding pertussis incidence and vaccination coverage. Historically, notification and hospitalisation rates for pertussis in Indigenous children have been three to eight times higher than those in non-Indigenous children across all age groups younger than 5 years of age.37
Vaccination coverage among Indigenous children has shown both progress and areas of concern. In 2022, the immunisation rate for Indigenous 1-year-olds was 91.1%, compared with 94.0% for non-Indigenous children. For 2-year-olds, the coverage was 89.1% among Indigenous children versus 92.2% among their non-Indigenous counterparts. However, at 5 years of age, Indigenous children had a higher coverage rate of 96.1%, surpassing the 94.1% observed in non-Indigenous children.55
The adolescent dose is seen as a crucial tool at reducing pertussis transmission to vulnerable infants. Despite its importance, the overall national coverage rate for adolescents turning 13 years of age who had received a booster dose declined from 76.2% in 2022 to 73.9% in 2023.56
Maternal vaccination plays a crucial role in protecting infants, who are at the highest risk for severe pertussis.57 However, vaccination rates among pregnant women have declined in Queensland, for instance, with vaccination rates dropping from 77.2% in 2020 to 70.7% in 2023.58
Pathogenesis and pathophysiology
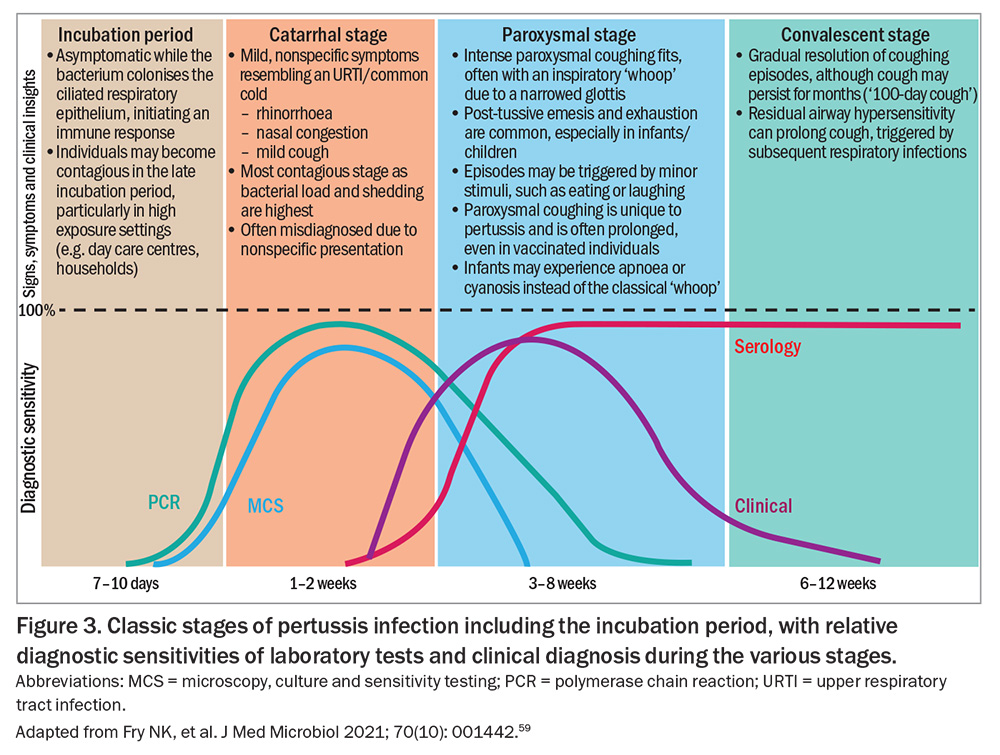
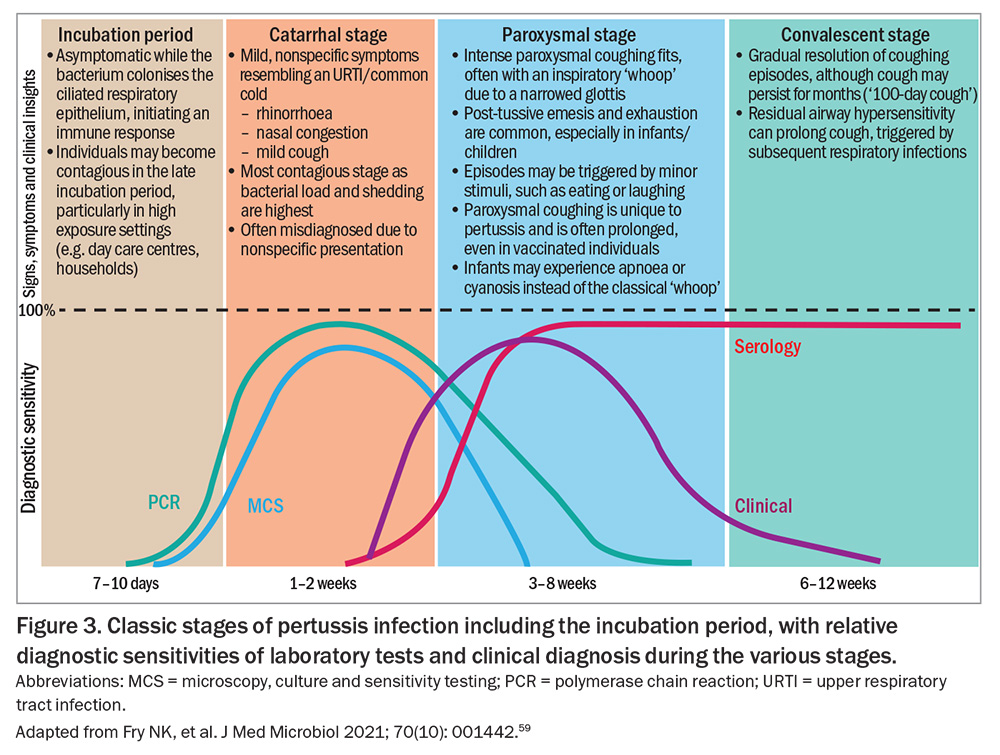
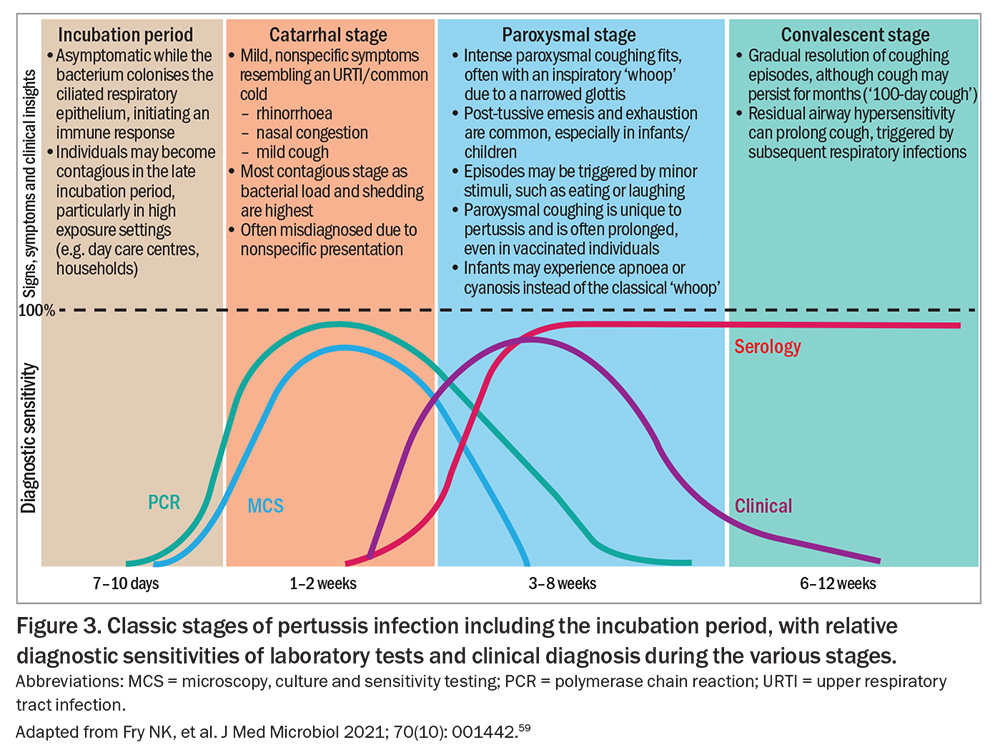
B. pertussis is highly adapted to the human respiratory tract. Infection is typically characterised by a prolonged illness, starting with an incubation period and progressing through three distinct clinical stages: catarrhal, paroxysmal and convalescent (Figure 3).59 Unlike many respiratory infections, pertussis is largely noninflammatory and does not typically induce fever, a feature that may help distinguish it from viral upper respiratory tract infections.2,60
The pathogenicity of the medically important Bordetella species is mainly attributed to the arsenal of virulence factors that facilitate attachment, immune evasion and damage to the host’s respiratory epithelium (Table 1).61-63
Attachment and colonisation
B. pertussis attaches to ciliated epithelial cells lining the respiratory tract. Adhesins such as PT, filamentous haemagglutinin (FHA), and fimbriae 2/3 (FIM2/3) facilitate this process. PT, an exotoxin unique to B. pertussis, also plays a crucial role in disease severity, particularly in infants.64,65 Antibodies to FIM protect against colonisation, and vaccines containing FIM2/3 antigens are associated with enhanced immunogenicity.66,67
Immune evasion
After attachment, Bordetella species deploy virulence factors to impair host defences. For B. pertussis, PT modifies G-protein signalling in host cells, leading to a cascade of effects, including lymphocytosis, impaired immune cell migration and altered cytokine responses.17 Adenylate cyclase toxin intoxicates neutrophils and macrophages by increasing intracellular cyclic adenosine monophosphate, disrupting their immune function.2 This helps B. pertussis, B. parapertussis and B. bronchiseptica evade immune clearance. PRN also confers resistance to neutrophil-mediated clearance.68 PRN-specific antibodies are highly protective, but the emergence of PRN-deficient strains caused by aP vaccine-driven selection pressure and antigenic variations poses a challenge for vaccine efficacy.27,68
Local and systemic effects
Tracheal cytotoxin damages ciliated epithelial cells, impairing mucociliary clearance.61 This contributes to, but does not cause, the characteristic paroxysmal cough. PT induces lymphocytosis by inhibiting lymphocyte migration and function, a hallmark of pertussis in infants and young children.17,64 In severe cases, leucocytosis may obstruct pulmonary vasculature, leading to pulmonary hypertension.70
Paroxysmal cough and ‘whoop’
The hallmark paroxysmal cough arises from persistent stimulation of sensory nerves in the respiratory tract, likely mediated by prolonged exposure to bacterial toxins and inflammatory mediators including bradykinin.71 The characteristic whoop occurs during the inspiratory phase following a prolonged coughing spell and results from forced inhalation through narrowed airways.
Complications in severe cases
Infants are particularly vulnerable to complications such as apnoea, cyanosis and secondary bacterial pneumonia. Severe pulmonary hypertension, often mediated by extreme leucocytosis, can lead to cardiac failure in young infants.70
Clinical features
Classic stages
B. pertussis infection follows a distinct clinical progression (Figure 3). The illness typically has a seven- to 10-day incubation period (range: six to 20 days). However, household exposure studies have shown that 22% of secondary cases can occur more than 28 days after symptom onset in the index case, especially when the infector is the father or sibling.72,73
Clinical manifestations usually last six to 12 weeks, although the timeline and severity may vary by age, vaccination status and immune response.2
Atypical presentations
Deviations from the classic pertussis presentation are seen throughout the age spectrum, especially when caused by B. parapertussis, B. bronchiseptica and B. holmesii.74
Infants (younger than 6 months of age)
Infants often present with atypical features compared with older children, making early diagnosis challenging. Rather than the characteristic paroxysmal cough with a whoop, infants, especially those younger than 3 months of age, often exhibit apnoea, cyanosis and feeding difficulties as their primary symptoms.2,75 These manifestations occur because of immature respiratory control mechanisms and increased susceptibility to PT-induced immune dysregulation. In some cases, the absence of an overt cough can further delay recognition of the disease.
Infants are at the highest risk of severe complications, including pneumonia, seizures, encephalopathy and respiratory failure, often requiring hospitalisation.76 The high mortality rate in this age group underscores the importance of early diagnosis, prompt antibiotic treatment and maternal immunisation during pregnancy to provide passive immunity.
Adolescents and adults
Adolescents and adults with pertussis often develop a prolonged cough rather than the classic whooping cough.77 The illness may present as a persistent dry cough lasting weeks to months, often leading to misdiagnosis as post-viral cough, asthma exacerbation or gastro-oesophageal reflux disease. Although the paroxysmal coughing episodes characteristic of pertussis may still occur, the inspiratory whoop is typically absent in these age groups.
Because of waning immunity, older children, adolescents and adults serve as reservoirs for transmission, particularly to vulnerable populations such as infants and immunocompromised individuals.38,39 Although complications are generally less severe than in infants, rib fractures, pneumothorax, subconjunctival haemorrhage, hernias and urinary incontinence can occur because of the forceful nature of paroxysmal coughing.2,47 The lack of awareness regarding pertussis in this age group can contribute to delayed presentation and diagnosis with increased transmission.
Vaccinated individuals
Individuals who have been previously vaccinated against pertussis can still become infected and transmit pertussis, often experiencing modified or milder disease.5 Although the classic paroxysmal cough and inspiratory whoop may still be present, the duration and intensity of symptoms tend to be less severe than in unvaccinated individuals.78 Post-tussive vomiting and complications occur less frequently.
Complications
Pertussis can lead to a range of complications, particularly in infants and in older people.
Respiratory complications
Pneumonia is a common and serious respiratory complication, caused by B. pertussis itself or secondary infection with other respiratory pathogens including Streptococcus pneumoniae, Haemophilus influenzae, respiratory syncytial virus and influenza A virus.20,79 In infants, pneumonia is a major contributor to pertussis-related morbidity and mortality, often requiring hospitalisation and respiratory support.
Another significant respiratory complication is atelectasis, which results from airway obstruction caused by excessive mucus production and impaired mucociliary clearance.2 In severe cases, respiratory distress and respiratory failure can develop, particularly in young infants who may require mechanical ventilation. Prolonged paroxysmal coughing episodes can also lead to pneumothorax or pneumomediastinum, particularly in older children and adults, because of increased intrathoracic pressure during coughing spasms.80
Neurological complications
Neurological complications of pertussis occur primarily in infants and are often secondary to hypoxaemia caused by prolonged coughing episodes or apnoea.4 Seizures are a common manifestation, occurring in about 4% of hospitalised infants with severe disease. Hypoxic encephalopathy and cerebral haemorrhage, both resulting from intense coughing bouts, can cause long-term neurodevelopmental impairments, including cognitive and motor deficits.81
Cardiovascular complications
Although cardiovascular complications are rare, they can occur in severe cases of pertussis, particularly in young infants with profound leucocytosis.70 Severe leucocytosis can lead to pulmonary hypertension, a potentially fatal complication resulting from vascular obstruction by excessive white blood cells.65 This condition can cause right heart failure and circulatory collapse, necessitating aggressive interventions such as exchange transfusion.
Older children and adults with pre-existing cardiovascular disease may experience arrhythmias or exacerbation of underlying heart conditions because of the physiological stress caused by prolonged coughing fits and oxygen desaturation.
Diagnosis
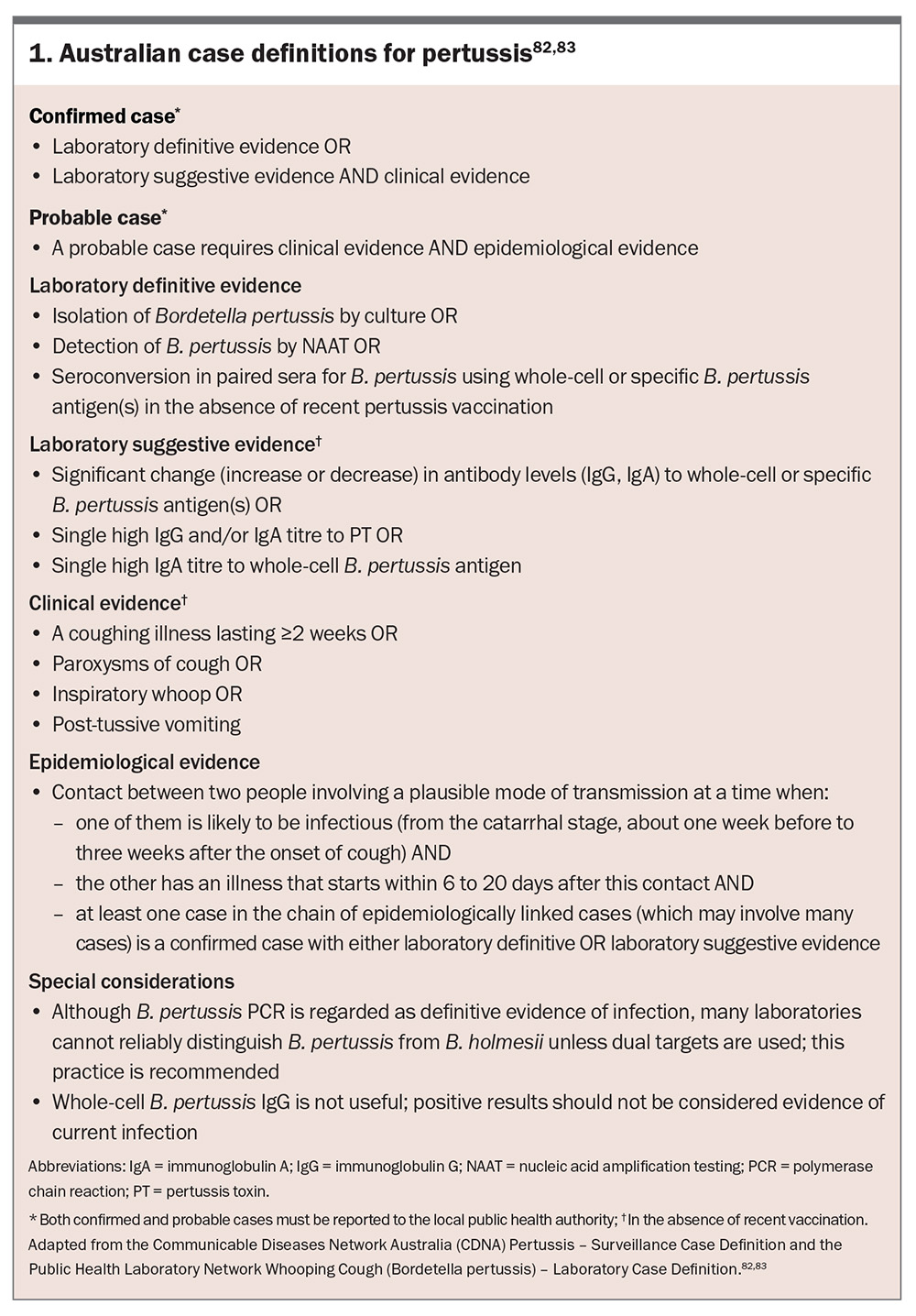
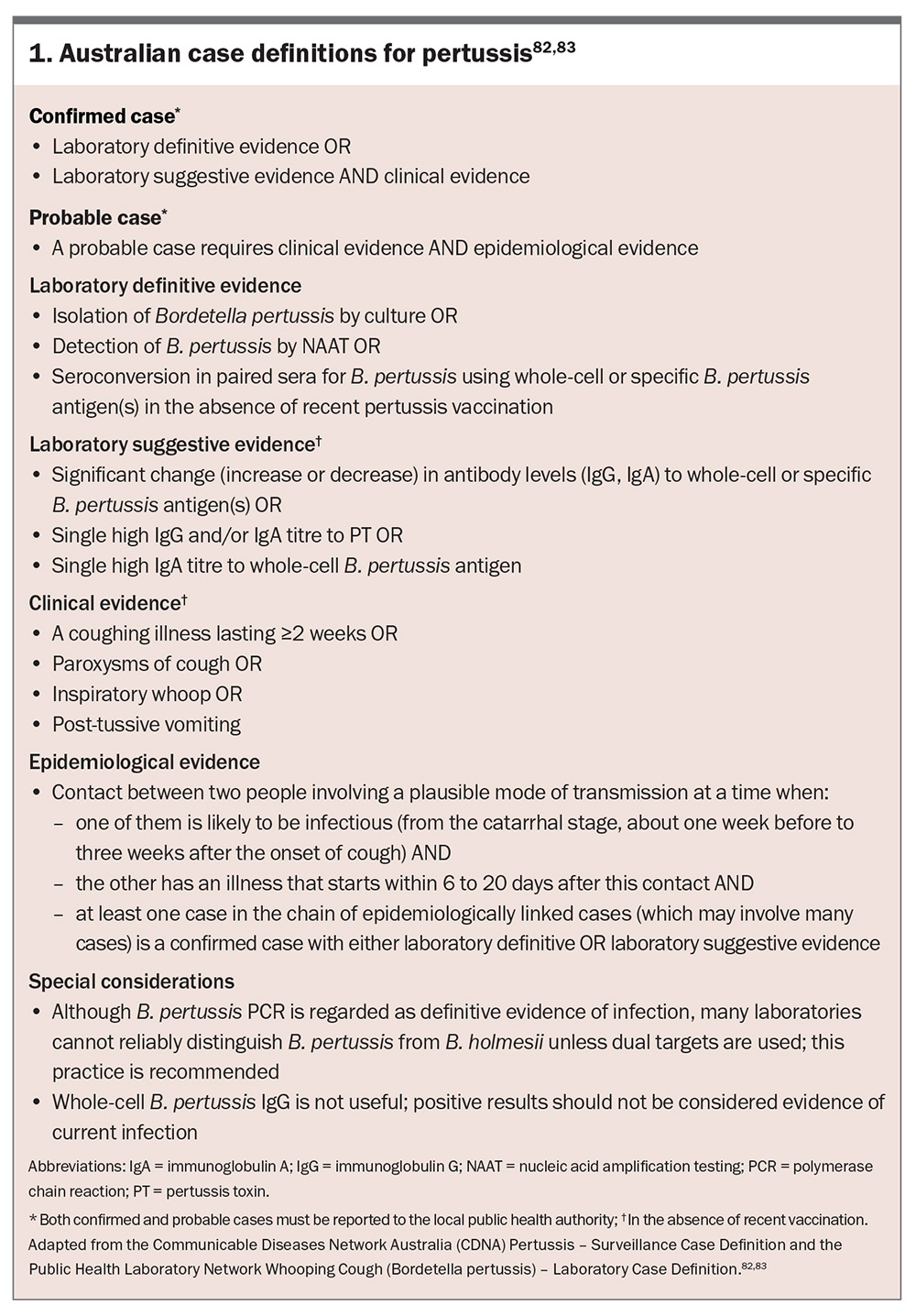
Early diagnosis of pertussis is crucial to mitigate disease transmission, initiate timely treatment and prevent complications, particularly in high-risk populations such as infants and in older people. However, pertussis can be challenging to diagnose because of its nonspecific early signs and symptoms, such as the common cold, and atypical presentations in vaccinated individuals. The establishment of pertussis surveillance and laboratory case definitions in Australia (Box 1) has provided a standardised framework for case identification, improving diagnostic consistency, outbreak detection and epidemiological tracking across diverse healthcare settings.82,83
Clinical diagnosis
High index of suspicion
A history of a coughing illness lasting two weeks or more, paroxysmal coughing spells, post-tussive vomiting or retching or inspiratory whoop strongly supports the clinical diagnosis of pertussis. Furthermore, recent contact with a confirmed or suspected pertussis case should be ascertained.
Limitations of clinical diagnosis
The reliance on clinical diagnosis alone, particularly in its early stages, can lead to delayed recognition, misdiagnosis and missed opportunities for timely intervention. In infants, adolescents and adults, classic features may be absent or muted, complicating clinical recognition. Furthermore, differentiation from other respiratory illnesses such as viral infections, asthma or bronchitis requires additional diagnostic investigation.
Laboratory diagnosis
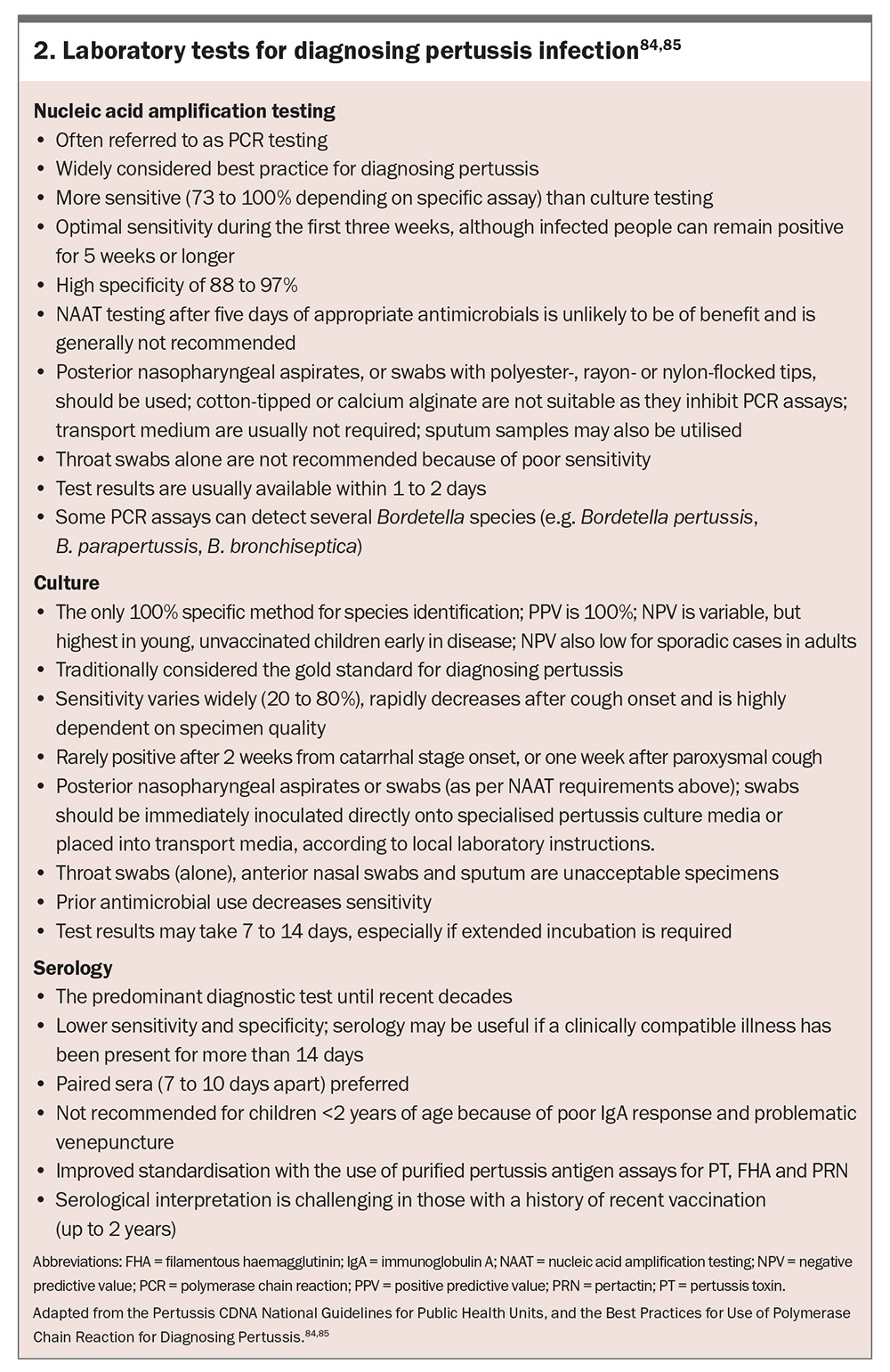
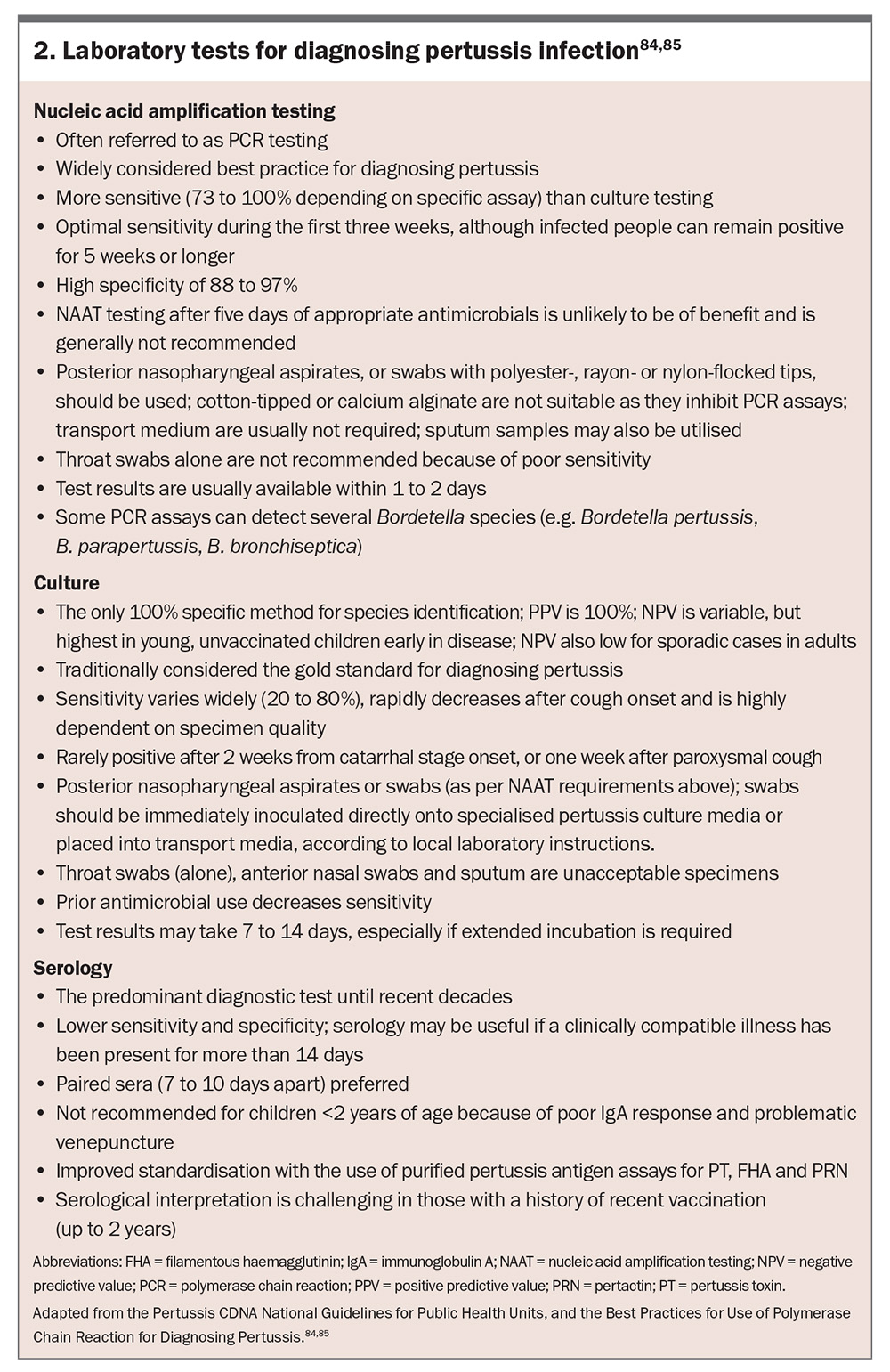
The duration of signs and symptoms at the time of presentation can help determine the optimal laboratory test (or combination) for diagnosis (Figure 3 and Box 2).59,84,85
Collection of specimens
Bordetella species can be isolated from posterior nasopharyngeal swabs, nasopharyngeal aspirates, throat swabs or induced sputa.86 Nasopharyngeal aspirates have shown a 15% gain in the isolation rate compared with nasopharyngeal swabs in infants.87 Given Bordetella species predominantly colonise and replicate in the ciliated epithelium of the nasopharynx, throat swabs or anterior nares (nasal)-only swabs are considered suboptimal and are not recommended.5,84
Polymerase chain reaction
Nucleic acid amplification testing by PCR is the preferred method for pertussis diagnosis during the first three to four weeks of illness.85,88 It detects specific DNA sequences for Bordetella species. Advantages include high sensitivity and specificity, rapid results and continued effectiveness even after antimicrobial initiation (although sensitivity may decrease).89 A potential limitation includes B. pertussis false-positives caused by cross-reactivity with other Bordetella species. For example, gene targets IS481 and IS1002 used for detecting B. pertussis show cross-reactivity with B. holmesii and certain B. bronchiseptica strains, and B. parapertussis, respectively.21,90 Reduced sensitivity is experienced later in the disease course. Furthermore, polyester, rayon or flocked nylon swabs are optimal for posterior nasopharyngeal collection, whereas cotton-tipped and calcium alginate swabs must not be used because they inhibit PCR assays.22,85
Microscopy, culture and sensitivity
Historically, culturing Bordetella species was considered the gold standard for diagnosis, offering 100% specificity for definitive confirmation and enabling antibiotic susceptibility testing; however, its routine use is limited because of several practical challenges.91-93 These include: a time-consuming process requiring seven to 12 days for results; the need for specialised media such as Regan-Lowe or Bordet-Gengou agar; difficulties in culturing B. holmesii because of the presence of cephalexin in selective media; and its low sensitivity.89 A flexible, flocked nylon swab with charcoal-containing media is the preferred posterior nasopharyngeal specimen to maximise yield.
Serology
Routine serological testing for the diagnosis of pertussis infection is not recommended as previous infection (including asymptomatic) and recent vaccination (past 12 months) can lead to inaccurate results.88 However, serological testing may be useful when the optimal timing for PCR and/or microscopy, culture and sensitivity testing has elapsed. Quantifying immunoglobulin G (IgG) antibodies to PT is the most used assay, and titres usually peak two to eight weeks after cough onset.22 Recent Australian data demonstrate that quantifying immunoglobulin A (IgA) antibodies to PT appears to contribute little diagnostic value to an accurate PT IgG assay.94 Advantages of serological testing include high sensitivity and specificity, rapid results and effectiveness even after antimicrobial initiation (although sensitivity may decrease).89 Important limitations include interpretation challenges because of prior vaccination and limited use in infants and young children.22
Differential diagnosis
Pertussis should be differentiated from other causes of prolonged cough and paroxysmal episodes, including:
- viral respiratory infections such as adenovirus, parainfluenza viruses and respiratory syncytial virus
- Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella species
- asthma exacerbation
- gastro-oesophageal reflux disease, which can mimic post-tussive vomiting
- Chlamydia trachomatis in neonates presenting with chronic cough without fever.
Utilising precollected nasopharyngeal swabs or nasopharyngeal aspirates, requesting a multiplex respiratory virus pathogen PCR panel (which typically includes influenza A and B, respiratory syncytial virus A and B, coronavirus [seasonal], severe acute respiratory syndrome coronavirus 2, parainfluenza viruses 1, 2, 3, and 4, human rhinovirus, enterovirus, adenovirus and human metapneumovirus), together with M. pneumoniae, C. pneumoniae and Legionella pneumophila PCR tests, may help to exclude other causes of acute respiratory illness. Additionally, a full blood count may reveal a lymphocytosis, particularly in infants during the early paroxysmal stage.95
Diagnostic implications for public health
Notification, reporting and surveillance
Pertussis, both confirmed and probable cases, is a notifiable disease in all Australian states and territories (Box 1 and Box 2).84 Prompt reporting ensures public health measures, including contact tracing and prophylaxis for exposed individuals, can be implemented. Accurate diagnosis supports epidemiological monitoring and informs vaccination strategies.88
Treatment in primary care
The primary objectives of pertussis treatment are to:
- prevent disease transmission, especially to vulnerable populations
- reduce the severity and duration of symptoms if commenced in the catarrhal stage, and
- minimise complications, such as pneumonia and acute respiratory distress syndrome.96
Antimicrobial therapy
Early initiation, ideally during the catarrhal stage when bacterial replication and shedding peaks, significantly reduces transmissibility.96 Antimicrobials are less effective in altering the disease course during the paroxysmal stage but are still recommended to prevent further spread.
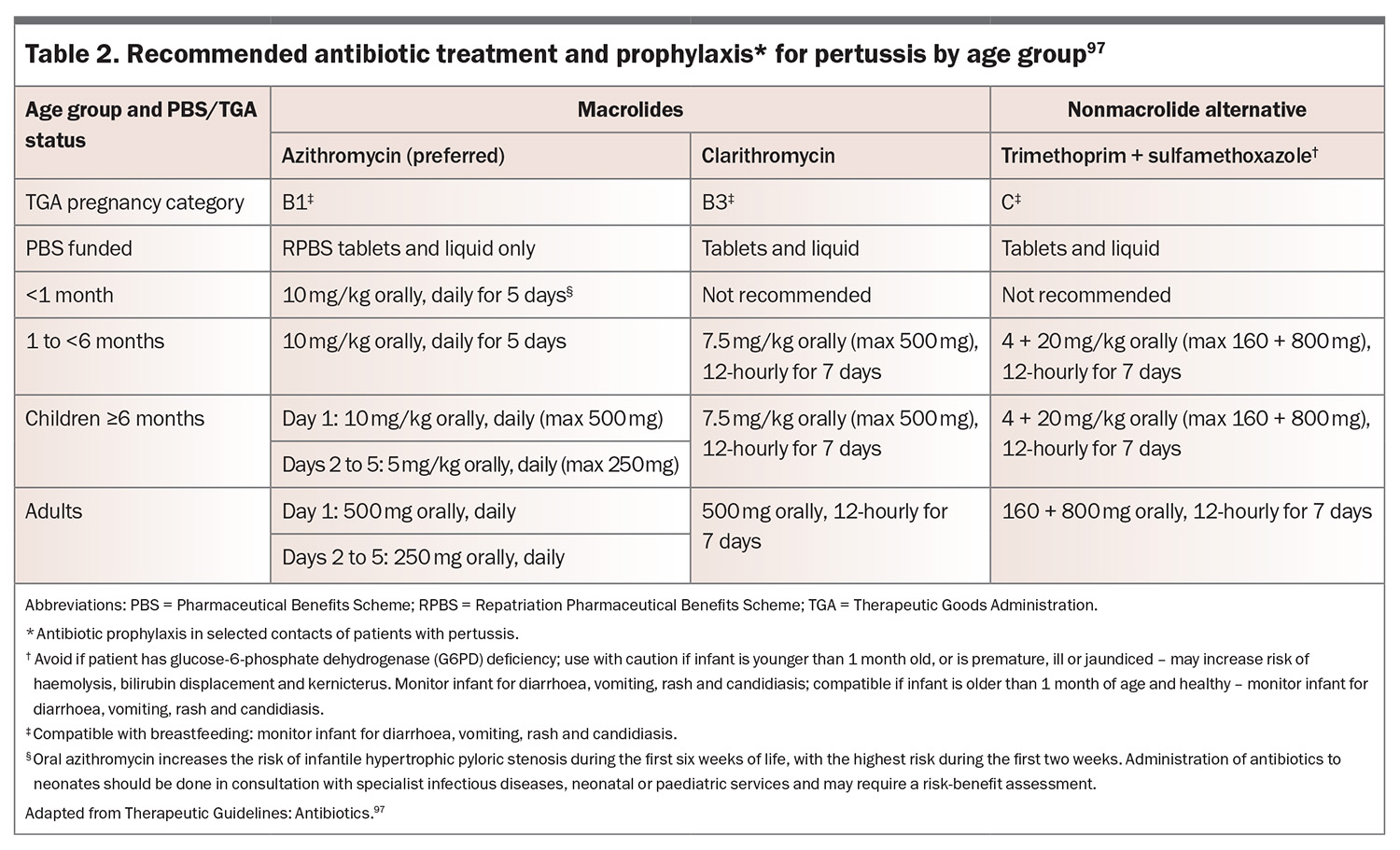
Macrolides as first-line agents
Patients infected with B. pertussis, B. parapertussis and B. holmesii should be treated with a macrolide antibiotic.2 Azithromycin is preferred because of its shorter course (five days) and better tolerability (Table 2).97 Clarithromycin is a suitable alternative, administered for seven days. Erythromycin is an effective agent but is associated with significant gastrointestinal side effects, limiting its use and is not normally recommended in Australia. There is no evidence to support the use of roxithromycin for the treatment of pertussis. Administration of antibiotics to neonates should be done in consultation with specialist infectious diseases, neonatal or paediatric services, especially given that macrolides are associated with infantile hypertrophic pyloric stenosis in this age group.84,98
Alternative antimicrobials
Trimethoprim-sulfamethoxazole is an option for patients who are intolerant to macrolides, have known or suspected macrolide resistance or are infected with B. bronchiseptica.2,97
Supportive care
Supportive measures are essential, particularly for infants and others with severe disease.
Symptom management
Cough suppressants are not recommended, as they do not alleviate the underlying cause of paroxysmal cough and may have adverse effects in children.99,100 Any form of sedation is reserved for inpatient management only.
Nutritional and hydration support
Frequent small feeds or nasogastric feeding may be necessary for infants and young children with feeding difficulties caused by paroxysmal coughing and vomiting. Intravenous fluids may be required in cases of significant dehydration or poor oral intake.
Respiratory support
Supplemental oxygen therapy is indicated for hypoxia or cyanotic episodes. Mechanical ventilation is required in cases of severe respiratory distress or apnoea, particularly in infants.
Postexposure prophylaxis
Indications and regimen
Timely initiation of postexposure prophylactic antimicrobials reduces the likelihood of spread to close contacts of confirmed pertussis cases, particularly household members (especially infants and pregnant women) and healthcare workers exposed to infectious patients.101 The same antimicrobials used for treatment are recommended for postexposure prophylaxis within 21 days of exposure to be effective in preventing secondary cases.97
Prevention
The primary aim of pertussis prevention is to reduce disease incidence, severity and transmission, especially among vulnerable populations such as infants. Strategies involve widespread vaccination, public health measures and education.88,102
Vaccination
Whole-cell pertussis and acellular pertussis vaccines
The wP vaccine was first introduced in the 1940s and consists of detoxified and heat-killed bacteria. It provides robust and long-lasting immunity to B. pertussis by inducing a strong Th1/Th17-driven immune response to more than 3000 antigens, mimicking natural infection.2 However, concerns over reactogenicity, including high rates of fever and injection site reactions, led to the development of the aP vaccine, subsequently replacing wP vaccination in most developed countries through the 1990s and 2000s.103
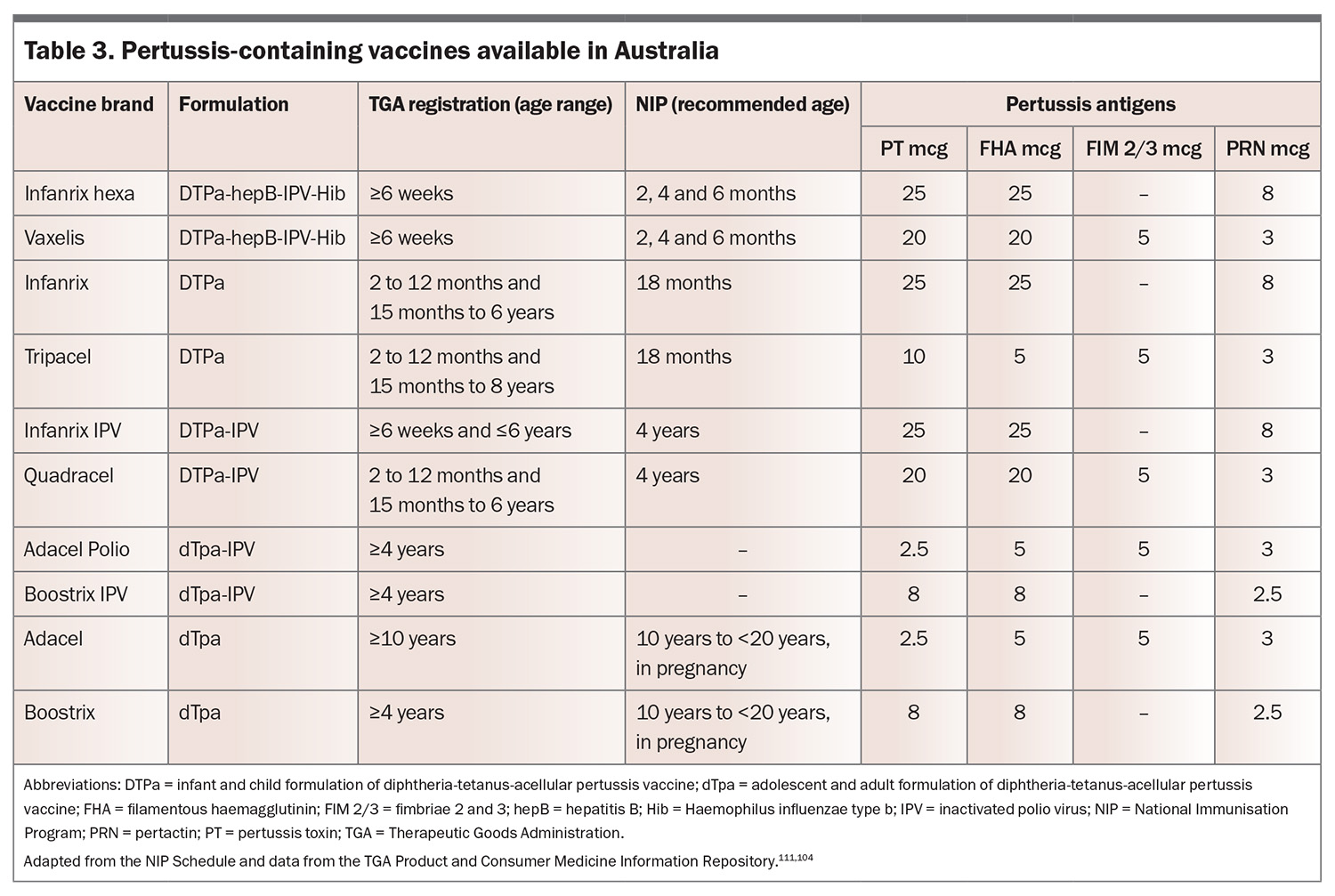
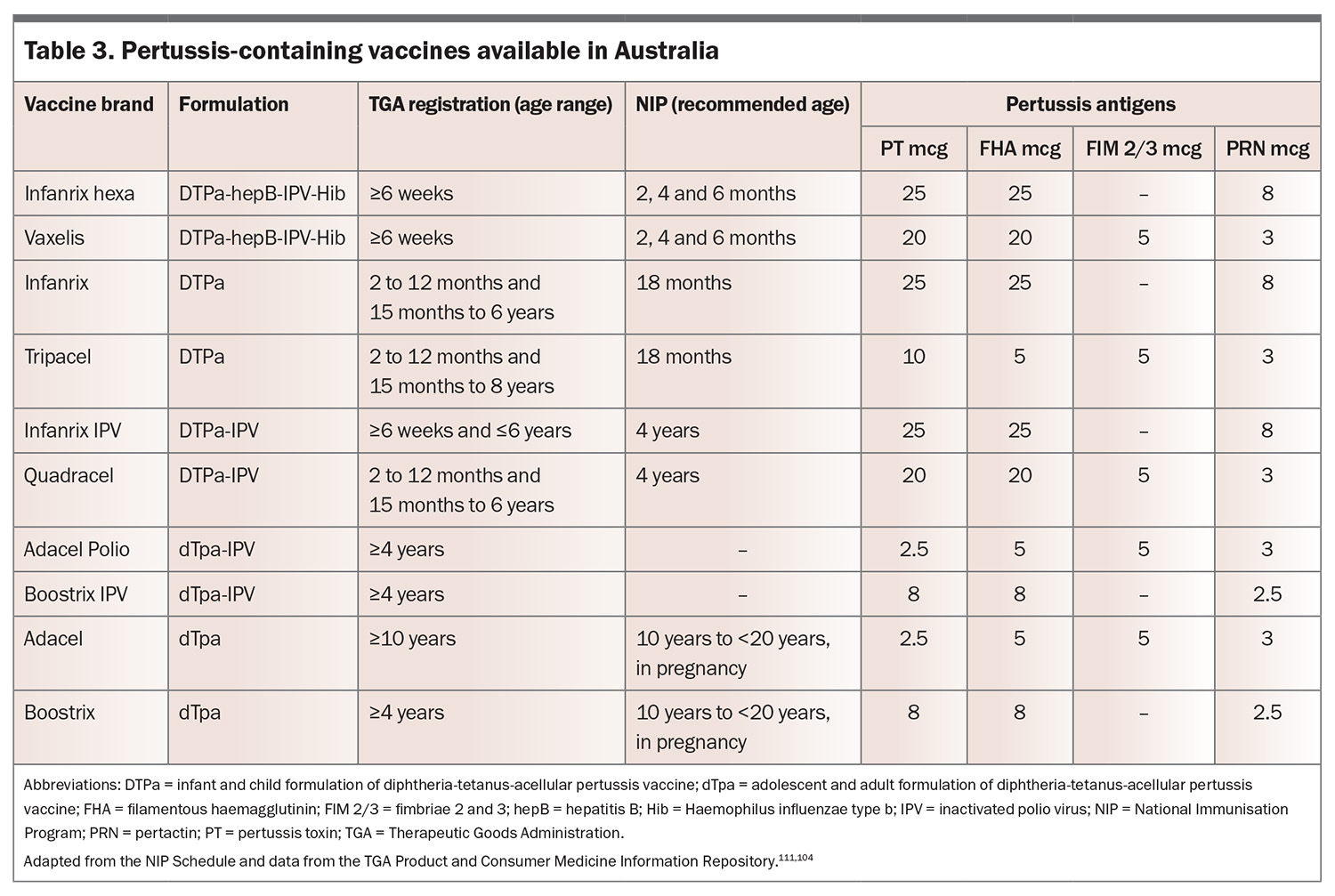
In Australia, the aP vaccine contains purified antigens to B. pertussis, typically including PT, FHA, PRN and FIM2/3 proteins (Table 3).104 These are combined with an aluminium-based adjuvant to enhance immune responses. Although the aP vaccine significantly decreases pertussis incidence, it induces a predominantly Th2-skewed immune response, which has been associated with reduced long-term protection compared with wP vaccines.42,50-52
Neither the wP nor the aP vaccines confer substantial cross-protection against B. parapertussis, B. bronchiseptica or B. holmesii.105,106 Studies suggest that prior vaccination with the aP vaccine may even facilitate B. parapertussis infection because of immune modulation that fails to provide sufficient clearance.107
Australian Immunisation Handbook
The Australian Immunisation Handbook (see: https://immunisationhandbook.health.gov.au) provides clinical advice on the safest and most effective use of vaccines.108 The handbook recommendations are developed by the Australian Technical Advisory Group on Immunisation and endorsed by the National Health and Medical Research Council. This online resource details information on vaccine preventable diseases and vaccinations for special risk groups.
The NIP funds many, but not all, of the vaccines. Individuals may receive, or be recommended to receive, vaccines described in the handbook that are not part of the routine immunisation schedule, such as those at occupational risk of disease, traveling overseas or who have a medical condition that puts them at increased risk of contracting a vaccine-preventable disease. The pertussis section within the handbook extensively references the NIP and the NIP Schedule.109-111
National Immunisation Program schedule
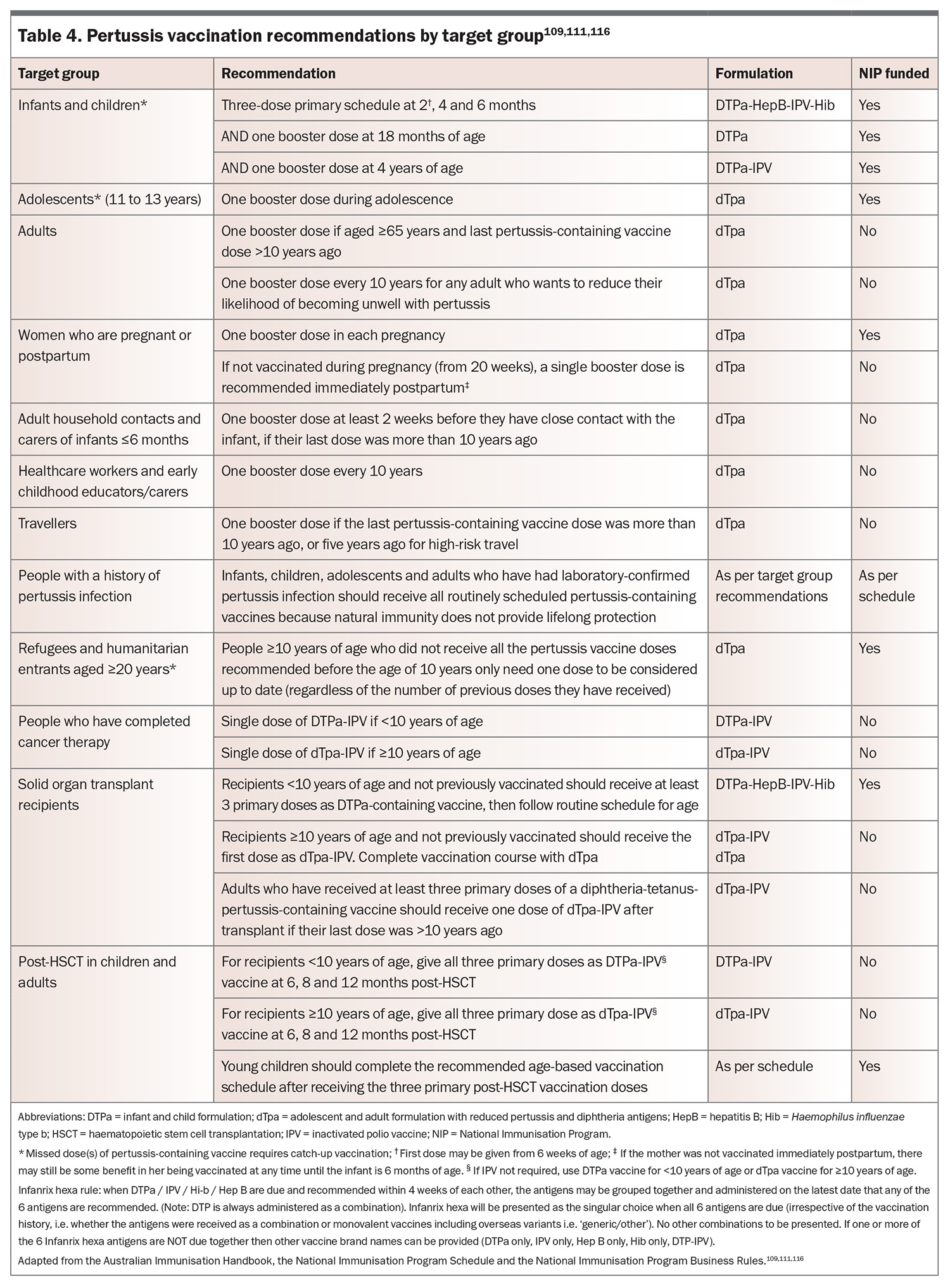
The NIP is a free, Commonwealth government-funded, state and territory government-delivered, program for certain immunisations administered at specific times from birth to adulthood for Medicare eligible individuals (Table 4).111 In Australia, vaccines containing pertussis are only available as combination vaccines (Table 3).
For pertussis, a primary three-dose schedule is administered at 2, 4 and 6 months of age, with single booster doses given at 18 months of age, and again at 4 years of age. Reduced-dose diphtheria-tetanus-acellular pertussis (dTpa) boosters are available for adolescents and pregnant women. When administered between 20 and 32 weeks’ gestation to optimise the transfer of maternal antibodies to the fetus, pertussis-containing immunisation provides passive immunity to infants during their first few months of life when they are most vulnerable. Importantly, recent Australian and international data confirm the effectiveness of this strategy, significantly reducing the risk of laboratory-confirmed disease among infants younger than 2 months of age.37,57,112-114
Refugees and humanitarian entrants aged 20 years or older can also get certain vaccines for free if they did not receive them in childhood. If a person has a record of vaccination from overseas, consider any previous doses when planning a catch-up vaccination schedule. Check the dosing intervals as some doses may be invalid because the interval between doses was too short. Check all vaccination records before vaccination as well because people may have visited multiple immunisation providers after they arrived in Australia.
There are no additional recommendations for Aboriginal and Torres Strait Islander children and adolescents.
Catch-up immunisation
Any immunisation given after the recommended age is a catch-up immunisation.115 Catch-up immunisations give patients their recommended vaccinations and help protect against disease by giving the best protection as fast as possible. This also includes individuals who have completed cancer therapy, have had a haemopoietic stem cell transplant, or are having/have had a solid organ transplant.116 The handbook includes a comprehensive National Immunisation Catch-up Calculator (available at: https://immunisationhandbook.health.gov.au/catch-up-calculator/calculator), enabling the production of an individualised catch-up vaccination schedule. People aged 20 years or older who have not received all vaccines may still benefit from a catch-up schedule. These are not funded under the NIP.
Cocooning strategy
Ensuring vaccination of close contacts (e.g. parents, household members, grandparents, caregivers) to create a protective ‘cocoon’ around infants is safe and effective in reducing transmission risk.117 Recent data demonstrated regular incomplete coverage, highlighting an area of focus when counselling expectant parents, especially fathers.118,119
Vaccination for healthcare workers
Healthcare workers can minimise transmission to vulnerable patients by receiving a pertussis-containing vaccine booster dose every 10 years.109
Vaccination for immunosuppressed patients
People with HIV, including pregnant women, can safely receive DTPa and dTpa (including when combined with H. influenzae type b and inactivated polio virus) vaccines according to routine recommendations.120-124 Because of immune suppression, the use of hepatitis B virus antigen-containing DTPa vaccines (DTPa-hepB-IPV-Hib) is not recommended, as higher strength hepatitis B formulations or increased doses may be required.
Herd immunity
High community vaccine coverage helps to reduce B. pertussis circulation and may indirectly protect unvaccinated individuals. Given that immunity to pertussis is not lifelong, regardless of natural infection or immunisation, regular booster dose coverage must remain consistently high to maintain herd immunity.52
Addressing vaccine hesitancy
Honest and transparent communication on vaccine benefits and safety profiles is a cornerstone of good medical practice. The complex issue of vaccine hesitancy, brought to the fore by COVID-19, may be approached through tailored messaging addressing specific concerns, including misinformation amplified by social media, to create a trusting environment for healthcare providers to advocate for vaccination.125-127
Public health measures
Surveillance, reporting and contact tracing
Enhanced surveillance ensures timely outbreak detection, case investigation, and implementation of control measures.84 With laboratory and clinician reporting, contact tracing can identify individuals exposed to confirmed cases and offer postexposure prophylaxis to limit secondary transmission, especially in high-risk community settings such as childcare facilities.
Education and awareness
Strategic community education campaigns help promote timely vaccination and recognition of pertussis symptoms.128,129 Frequent messaging for parents may further reinforce the importance of adhering to vaccination schedules.
Challenges to overcome
The resurgence of pertussis in Australia in the post-COVID-19 era highlights important challenges in controlling this largely vaccine-preventable disease. Despite high vaccination coverage achieved through the NIP, pertussis remains endemic, with periodic outbreaks occurring every three to five years pre-COVID-19. The decline in notifications observed during the pandemic because of widespread public health measures has been followed by a sharp rebound as these interventions have been lifted, underscoring the ongoing vulnerability of the population. This resurgence may be attributed, at least in part, to several factors, including epidemiological shifts such as genomic reshuffling, asymptomatic infection, waning immunity, declining immunisation uptake in some target groups, and increased transmission as social interactions normalised.
Given these challenges, a multifaceted approach is required to strengthen pertussis control in Australia. Enhancing vaccine strategies, including optimising booster schedules and investigating next-generation vaccines that elicit more durable immunity, is crucial. Maternal vaccination remains a highly effective strategy for protecting highly vulnerable infants, yet recent declines in uptake emphasise the need for continued public health messaging and provider advocacy. Additionally, improving access to vaccines among Indigenous and other high-risk populations must be prioritised to reduce health disparities.
Diagnostic vigilance is essential, particularly in recognising atypical presentations across age groups, including older individuals and vaccinated populations. Early identification through PCR-based laboratory testing remains the preferred method, given its high sensitivity in the first few weeks of illness. Given the current unprecedented levels of pertussis circulating throughout the Australian community, it is imperative that GPs and other frontline healthcare workers maintain a high index of suspicion for pertussis, particularly in cases of prolonged cough or exposure to infected individuals. This improved clinical awareness, combined with prompt reporting and contact tracing, is vital for timely intervention and outbreak mitigation.
Public health efforts should also focus on addressing vaccine hesitancy, which has been exacerbated by misinformation and pandemic-related disruptions. Strengthening communication strategies to reinforce vaccine safety and effectiveness are important to improve public confidence in immunisation programs. Enhanced surveillance systems, including genomic monitoring of circulating B. pertussis strains and improving diagnostic data collection, will aid in assessing vaccine effectiveness, improve outbreak response and inform future immunisation policies.
Conclusion
The unique pathophysiology of pertussis explains its protracted course and characteristic clinical features. Understanding the mechanisms of immune evasion and the role of toxin-mediated damage is essential for timely diagnosis, effective treatment and targeted prevention strategies. Recognising the pathophysiological underpinnings can help clinicians anticipate complications, particularly in high-risk groups such as infants and immunocompromised individuals.
The role of GPs in recognising, treating and preventing pertussis cannot be overstated. By staying informed of evolving epidemiological trends and clinical guidelines, healthcare providers can play a pivotal role in reducing the burden of pertussis and protecting vulnerable populations.
As Australia transitions into the post-COVID-19 era, the lessons learned during the pandemic, such as the importance of public health infrastructure and community engagement, must be applied to strengthen pertussis control efforts. With sustained vigilance and innovation, the goal of reducing pertussis-related morbidity and mortality remains achievable. RMT
COMPETING INTERESTS: Professor Griffin has received speaker honoraria from Seqirus. Dr Armstrong and Dr Spence: None.
ACKNOWLEDGEMENTS: The authors would like to thank the reviewers for their considered thoughts and recommendations, strengthening this paper’s readability and applicability.
References
1. Bordet J, Gengou O. Le microbe de la coqueluche (The whooping cough microbe). Ann Inst Pasteur (Paris) 1906; 20: 731-741.
2. Cherry JD, Heininger U. Pertussis and other Bordetella infections. In: Cherry JD, Kaplan SL, Harrison GJ, Steinbach WJ, Hotez PJ, Williams JV, eds. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 9th ed. Elsevier; 2025: 1206-1224. e12.
3. Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 2014; 12: 274-288.
4. Souder EE, Long SS. Pertussis (Bordetella pertussis and Bordetella parapertussis). In: Kliegman R, St. Geme III JW, Blum NJ, et al, eds. Nelson Textbook of Pediatrics. 22nd ed. 2025: 1761-1765.e1.
5. Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 2005; 18: 326-382.
6. Cherry JD, Seaton BL. Patterns of Bordetella parapertussis respiratory illnesses: 2008-2010. Clin Infect Dis 2012; 54: 534-537.
7. Noble BA, Jiudice SS, Jones JD, Timbrook TT. Reemergence of Bordetella parapertussis, United States, 2019-2023. Emerg Infect Dis 2024; 30: 1058-1060.
8. Heininger U, Stehr K, Schmitt-Grohe S, et al. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr Infect Dis J 1994; 13: 306-309.
9. Vindenes T, Gillespie WB, Gawoski J, Ooi WW, Wener K. Kennel cough in a dog and his best friend: Bordetella bronchiseptica causing pneumonia transmitted from dog and a review of the literature. Infect Dis Clin Pract (Baltim Md) 2015; 23: 118-122.
10. Miguelena Chamorro B, De Luca K, Swaminathan G, Longet S, Mundt E, Paul S. Bordetella bronchiseptica and Bordetella pertussis: similarities and differences in infection, immuno-modulation, and vaccine considerations. Clin Microbiol Rev 2023; 36: e0016422.
11. Woolfrey BF, Moody JA. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev 1991; 4: 243-255.
12. Fong W, Timms V, Holmes N, Sintchenko V. Detection and incidence of Bordetella holmesii in respiratory specimens from patients with pertussis-like symptoms in New South Wales, Australia. Pathology 2018; 50: 322-326.
13. Hamidou Soumana I, Linz B, Harvill ET. Environmental origin of the genus Bordetella. Front Microbiol 2017; 8: 28.
14. Muloiwa R, Dube FS, Nicol MP, Hussey GD, Zar HJ. Co-detection of Bordetella pertussis and other respiratory organisms in children hospitalised with lower respiratory tract infection. Sci Rep. 2020; 10: 16412.
15. Frassanito A, Nenna R, Nicolai A, et al. Infants hospitalized for Bordetella pertussis infection commonly have respiratory viral coinfections. BMC Infect Dis 2017; 17: 492.
16. Bridel S, Bouchez V, Brancotte B, et al. A comprehensive resource for Bordetella genomic epidemiology and biodiversity studies. Nat Commun 2022; 13: 3807.
17. Carbonetti NH. Contribution of pertussis toxin to the pathogenesis of pertussis disease. Pathog Dis 2015; 73(8): ftv073.
18. Parkhill J, Sebaihia M, Preston A, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 2003; 35: 32-40.
19. Bryant J, Chewapreecha C, Bentley SD. Developing insights into the mechanisms of evolution of bacterial pathogens from whole-genome sequences. Future Microbiol 2012; 7: 1283-1296.
20. Waters V, Halperin SA. Bordetella pertussis. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. 2020: 2793-2802.e3.
21. Yee R. Do not let perfect be the enemy of good: the current reality of Bordetella testing. Microbiol Spectr 2025; 13(1): e0221024.
22. European Centre for Disease Prevention and Control (ECDC). Laboratory Diagnosis and Molecular Surveillance of Bordetella Pertussis. ECDC; 2022. 37p.
23. Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH. Complexity of the basic reproduction number (R0). Emerg Infect Dis 2019; 25: 1-4.
24. Anderson RM, May RM. Directly transmitted infections diseases: control by vaccination. Science 1982; 215: 1053-1060.
25. Kretzschmar M, Teunis PF, Pebody RG. Incidence and reproduction numbers of pertussis: estimates from serological and social contact data in five European countries. PLoS Med 2010; 7(6): e1000291.
26. Gambhir M, Clark TA, Cauchemez S, Tartof SY, Swerdlow DL, Ferguson NM. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput Biol 2015; 11(4): e1004138.
27. Jayasundara D, Lee E, Octavia S, Lan R, Tanaka MM, Wood JG. Emergence of pertactin-deficient pertussis strains in Australia can be explained by models of vaccine escape. Epidemics. 2020; 31: 100388
28. Leong RNF, Wood JG, Turner RM, Newall AT. Estimating seasonal variation in Australian pertussis notifications from 1991 to 2016: evidence of spring to summer peaks. Epidemiol Infect 2019; 147: e155.
29. Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect 2014; 142: 672-684.
30. van der Kooi S, Beard F, Dey A, McIntyre P, Imai C, Amin J. Pertussis notifications decline in Australia during COVID-19 non-pharmaceutical interventions, 2020-2021. Commun Dis Intell (2018). 2024; 48.
31. Bricks LF, Vargas-Zambrano JC, Macina D. Epidemiology of pertussis after the COVID-19 pandemic: analysis of the factors involved in the resurgence of the disease in high-, middle-, and low-income countries. Vaccines (Basel) 2024; 12(12) 1346.
32. Khalil A, Samara A, Campbell H, Ladhani SN, Amirthalingam G. Recent increase in infant pertussis cases in Europe and the critical importance of antenatal immunizations: We must do better...now. Int J Infect Dis 2024; 146: 107148.
33. Australian Goverment Department of Health and Aged Care. National Communicable Disease Surveillance Dashboard. National Notifiable Diseases Surveillance System (NNDSS). Commonwealth of Australia. Updated March 1, 2025. Available online at: https://nindss.health.gov.au/pbi-dashboard/ (accessed March 2025).
34. Nie Y, Zhang Y, Yang Z, et al. Global burden of pertussis in 204 countries and territories, from 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Public Health. 2024; 24: 1453.
35. Kandeil W, van den Ende C, Bunge EM, Jenkins VA, Ceregido MA, Guignard A. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vaccines 2020; 19: 621-638.
36. Zhang S, Wang S, Liu J. Global, regional and national trends in incidence and mortality of pertussis from 1990 to 2021 and the comparison before and during COVID-19: a modelling analysis. J Infect Public Health 2025; 18: 102696.
37. Marshall KS, Quinn HE, Pillsbury AJ, et al. Australian vaccine preventable disease epidemiological review series: Pertussis, 2013-2018. Commun Dis Intell (2018). 2022; 46.
38. Bertilone C, Wallace T, Selvey LA. Finding the ‘who’ in whooping cough: vaccinated siblings are important pertussis sources in infants 6 months of age and under. Commun Dis Intell Q Rep 2014; 38: E195-200. Available online at: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3803c.htm (accessed March 2025).
39. Kardos P, Correia de Sousa J, Heininger U, et al. Understanding the impact of adult pertussis and current approaches to vaccination: a narrative review and expert panel recommendations. Hum Vaccin Immunother 2024; 20: 2324547.
40. Chuk LM, Lambert SB, May ML, et al. Pertussis in infants: how to protect the vulnerable? Commun Dis Intell Q Rep 2008; 32: 449-456.
41. Warfel JM, Beren J, Merkel TJ. Airborne transmission of Bordetella pertussis. J Infect Dis 2012; 206: 902-906.
42. Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA 2014; 111: 787-792.
43. Trainor EA, Nicholson TL, Merkel TJ. Bordetella pertussis transmission. Pathog Dis 2015; 73: ftv068.
44. Domenech de Celles M, Rohani P. Pertussis vaccines, epidemiology and evolution. Nat Rev Microbiol 2024; 22: 722-735.
45. Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med 2015; 13: 146.
46. Craig R, Kunkel E, Crowcroft NS, et al. Asymptomatic infection and transmission of pertussis in households: a systematic review. Clin Infect Dis 2020; 70: 152-161.
47. Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr 2016; 4(3): ei10-0008-2015.
48. Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc Biol Sci 2010; 277: 3239-3245.
49. Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science 2010; 330: 982-985.
50. Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 2012; 367: 1012-1019.
51. Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol 2013; 6(4): 787-796.
52. Szwejser-Zawislak E, Wilk MM, Piszczek P, Krawczyk J, Wilczynska D, Hozbor D. Evaluation of whole-cell and acellular pertussis vaccines in the context of long-term herd immunity. Vaccines (Basel) 2022; 11(1): 1.
53. Xu Z, Hu D, Luu LDW, et al. Genomic dissection of the microevolution of Australian epidemic Bordetella pertussis. Emerg Microbes Infect 2022; 11: 1460-1473.
54. Clarke M, McIntyre PB, Blyth CC, et al. The relationship between Bordetella pertussis genotype and clinical severity in Australian children with pertussis. J Infect 2016; 72: 171-178.
55. Australian Institute of Health and Welfare (AIHW). Immunisation and Vaccination. Commonwealth of Australia Department of Health and Aged Care. Updated June 17, 2024. Available online at: https://www.aihw.gov.au/reports/australias-health/immunisation-and-vaccination (accessed March 2025).
56. Hull B, Hendry A, Macartney K, Beard F. Annual Immunisation Coverage Report 2023. National Centre for Immunisation Research and Surveillance (NCIRS); 2024. 101p. Available online at: https://ncirs.org.au/annual-immunisation-coverage-report-2023 (accessed March 2025).
57. Regan AK, Moore HC, Binks MJ, et al. Maternal pertussis vaccination, infant immunization, and risk of pertussis. Pediatrics 2023; 152(5): e2023062664.
58. Queensland Department of Health. Declining Vaccination, Rising Whooping Cough Risk. Press release. The State of Queensland; November 25, 2024. Available online at: https://www.health.qld.gov.au/newsroom/doh-media-releases/declining-vaccination,-rising-whooping-cough-risk#:~:text=This%20call%20to%20action%20comes,and%20Cape%20and%20West%20Moreton (accessed March 2025).
59. Fry NK, Campbell H, Amirthalingam G. JMM Profile: Bordetella pertussis and whooping cough (pertussis): still a significant cause of infant morbidity and mortality, but vaccine-preventable. J Med Microbiol 2021; 70(10): 001442.
60. Cherry JD, Paddock CD. Pathogenesis and histopathology of pertussis: implications for immunization. Expert Rev Vaccines 2014; 13: 1115-1123.
61. Belcher T, Dubois V, Rivera-Millot A, Locht C, Jacob-Dubuisson F. Pathogenicity and virulence of Bordetella pertussis and its adaptation to its strictly human host. Virulence 2021; 12: 2608-2632.
62. Scanlon K, Skerry C, Carbonetti N. Role of major toxin virulence factors in pertussis infection and disease pathogenesis. Adv Exp Med Biol 2019; 1183: 35-51.
63. Linz B, Ivanov YV, Preston A, et al. Acquisition and loss of virulence-associated factors during genome evolution and speciation in three clades of Bordetella species. BMC Genomics 2016; 17: 767.
64. Carbonetti NH, Artamonova GV, Mays RM, Worthington ZE. Pertussis toxin plays an early role in respiratory tract colonization by Bordetella pertussis. Infect Immun 2003; 71: 6358-6366.
65. Paddock CD, Sanden GN, Cherry JD, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis 2008; 47: 328-338.
66. Dorji D, Mooi F, Yantorno O, Deora R, Graham RM, Mukkur TK. Bordetella pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med Microbiol Immunol 2018; 207: 3-26.
67. Queenan AM, Dowling DJ, Cheng WK, et al. Increasing FIM2/3 antigen-content improves efficacy of Bordetella pertussis vaccines in mice in vivo without altering vaccine-induced human reactogenicity biomarkers in vitro. Vaccine 2019; 37: 80-89.
68. Ma L, Caulfield A, Dewan KK, Harvill ET. Pertactin-deficient Bordetella pertussis, vaccine-driven evolution, and reemergence of pertussis. Emerg Infect Dis 2021; 27: 1561-1566.
69. Heininger U, Martini H, Eeuwijk J, et al. Pertactin deficiency of Bordetella pertussis: insights into epidemiology, and perspectives on surveillance and public health impact. Hum Vaccin Immunother 2024; 20: 2435134.
70. Cherry JD. The prevention of severe pertussis and pertussis deaths in young infants. Expert Rev Vaccines 2019; 18: 205-208.
71. Horiguchi Y. Current understanding of Bordetella-induced cough. Microbiol Immunol 2024; 68: 123-129.
72. Heininger U, Cherry JD, Stehr K, et al. Comparative efficacy of the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine and Lederle whole-cell component DTP vaccine in German children after household exposure. Pediatrics 1998; 102(3 Pt 1): 546-553.
73. Te Beest DE, Henderson D, van der Maas NA, et al. Estimation of the serial interval of pertussis in Dutch households. Epidemics 2014; 7: 1-6.
74. Valero-Rello A, Henares D, Acosta L, et al. Validation and implementation of a diagnostic algorithm for DNA detection of Bordetella pertussis, B. parapertussis, and B. holmesii in a pediatric referral hospital in Barcelona, Spain. J Clin Microbiol 2019; 57(1): e01231-18.
75. Winter K, Zipprich J, Harriman K, et al. Risk factors associated with infant deaths from pertussis: a case-control study. Clin Infect Dis 2015; 61: 1099-1106.
76. Mbayei SA, Faulkner A, Miner C, et al. Severe pertussis infections in the United States, 2011-2015. Clin Infect Dis 2019; 69: 218-226.
77. Senzilet LD, Halperin SA, Spika JS, et al. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clin Infect Dis 2001; 32: 1691-1697.
78. Havers FP, Moro PL, Hariri S, Skoff T, US Centers for Disease Control and Prevention (CDC). Pertussis. In: Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. Public Health Foundation; 2021. Last updated April 12, 2024. Available online at: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-16-pertussis.html (accessed March 2025).
79. Beynon KA, Young SA, Laing RT, Harrison TG, Anderson TP, Murdoch DR. Bordetella pertussis in adult pneumonia patients. Emerg Infect Dis 2005; 11: 639-641.
80. Long SS, Edwards KM, Mertsola J. Bordetella pertussis (Pertussis) and other Bordetella species. In: Long SS, Prober CG, Fischer M, Kimberlin D, eds. Principles and Practice of Pediatric Infectious Diseases. 6th ed. Elsevier; 2023: 909-918.e4.
81. Decker MD, Edwards KM. Pertussis (whooping cough). J Infect Dis 2021; 224(12 Suppl 2): S310-S320.
82. Communicable Diseases Network Australia (CDNA). Pertussis – Surveillance Case Definition. Commonwealth of Australia Department of Health and Aged Care; 2004. 2p. Available online at: https://www.health.gov.au/sites/default/files/documents/2022/06/pertussis-whooping-cough-surveillance-case-definition.pdf (accessed March 2025).
83. Public Health Laboratory Network. Whooping Cough (Bordetella pertussis) – Laboratory Case Definition. Commonwealth of Australia Department of Health and Aged Care; 2011. 14p. Available online at: https://www.health.gov.au/sites/default/files/documents/2022/06/whooping-cough-laboratory-case-definition.pdf (accessed March 2025).
84. Australian Centre for Disease Control. Pertussis CDNA National Guidelines for Public Health Units (version 4.0, October 2024). Commonwealth of Australia Department of Health and Aged Care; 2024. 43p. Available online at: https://www.health.gov.au/sites/default/files/2024-10/pertussis-whooping-cough-cdna-national-guidelines-for-public-health-units.pdf (accessed March 2025).
85. US Centers for Disease Control and Prevention (CDC). Best Practices for Use of Polymerase Chain Reaction for Diagnosing Pertussis. US Department of Health and Human Services. Updated April 2, 2024. Available online at: https://www.cdc.gov/pertussis/php/pcr-bestpractices/index.html (accessed March 2025).
86. Nunes MC, Soofie N, Downs S, et al. Comparing the yield of nasopharyngeal swabs, nasal aspirates, and induced sputum for detection of Bordetella pertussis in hospitalized infants. Clin Infect Dis 2016; 63(suppl 4): S181-S186.
87. World Health Organization (WHO). Laboratory Manual for the Diagnosis of Whooping Cough Caused by Bordetella pertussis/Bordetella parapertussis: Updated 2014. WHO; 2014. 49p. Available online at: https://www.who.int/publications/i/item/WHO-IVB-14.03 (accessed March 2025).
88. World Health Organization (WHO). Pertussis: Vaccine Preventable Diseases Surveillance Standards. WHO; 2018. 16p. Available online at: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-pertussis (accessed March 2025).
89. van der Zee A, Schellekens JF, Mooi FR. Laboratory diagnosis of pertussis. Clin Microbiol Rev 2015; 28: 1005-1026.
90. Cole M, Simon AK, Faulkner A, et al. Comparison of Bordetella species identification among differing rt-PCR assays in the United States. Microbiol Spectr 2024; 12(8): e0078324.
91. Kaczmarek MC, Valenti L, Kelly HA, Ware RS, Britt HC, Lambert SB. Sevenfold rise in likelihood of pertussis test requests in a stable set of Australian general practice encounters, 2000-2011. Med J Aust 2013; 198: 624-628.
92. Lee AD, Cassiday PK, Pawloski LC, et al. Clinical evaluation and validation of laboratory methods for the diagnosis of Bordetella pertussis infection: culture, polymerase chain reaction (PCR) and anti-pertussis toxin IgG serology (IgG-PT). PLoS One 2018; 13(4): e0195979.
93. European Centre for Disease Prevention and Control (ECDC). External quality assessment scheme for Bordetella pertussis antimicrobial susceptibility testing, 2022. ECDC; 2023. 29p.
94. May ML, Evans J, Holgate T, Doi SA, Ross P, Robson JM. Pertussis toxin IgA testing over-diagnoses recent pertussis infection. Pathology 2017; 49: 770-775.
95. Cherry JD. Pertussis in young infants throughout the world. Clin Infect Dis 2016; 63(Suppl 4): S119-S122.
96. Kilgore PE, Salim AM, Zervos MJ, Schmitt HJ. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev 2016; 29: 449-486.
97. Antibiotic Expert Group. Antibiotics. Therapeutic Guidelines. Therapeutic Guidelines Ltd; 2019. Available online at: https://tgldcdp.tg.org.au/guideLine?guidelinePage=Antibiotic&frompage=etgcomplete (accessed March 2025).
98. Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics 2015; 135: 483-488.
99. Sung V, Cranswick N. Cough and cold remedies for children. Aust Prescr 2009; 32: 122-124.
100. Wang K, Bettiol S, Thompson MJ, et al. Symptomatic treatment of the cough in whooping cough. Cochrane Database Syst Rev 2014; 2014(9): CD003257.
101. Britton P, Jones C. Pertussis prophylaxis. Aust Prescr 2012; 35: 82-84.
102. World Health Organization (WHO). Pertussis vaccines: WHO position paper – August 2015. Wkly Epidemiol Rec 2015; 90: 433-460. Available online at: https://www.who.int/publications/i/item/WHO-WER9035 (accessed March 2025).
103. Perez Chacon G, Estcourt MJ, Totterdell J, et al. Immunogenicity, reactogenicity, and IgE-mediated immune responses of a mixed whole-cell and acellular pertussis vaccine schedule in Australian infants: a randomised, double-blind, noninferiority trial. PLoS Med 2024; 21: e1004414.
104. Australian Goverment Department of Health and Aged Care. Therapeutic Goods Administration Product and Consumer Medicine Information Repository. eBusiness Services. Canberra: Commonwealth of Australia. March 1, 2025. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=pi&q=pertussis (accessed March 2025).
105. Zhang X, Weyrich LS, Lavine JS, Karanikas AT, Harvill ET. Lack of cross-protection against Bordetella holmesii after pertussis vaccination. Emerg Infect Dis 2012; 18: 1771-1779.
106. Rodgers L, Martin SW, Cohn A, et al. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis--Ohio, 2010-2011. Clin Infect Dis 2013; 56: 322-331.
107. Long GH, Karanikas AT, Harvill ET, Read AF, Hudson PJ. Acellular pertussis vaccination facilitates Bordetella parapertussis infection in a rodent model of bordetellosis. Proc Biol Sci. 2010; 277: 2017-2025.
108. Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook. Commonwealth of Australia Department of Health and Aged Care; 2025. Updated February 7, 2025. Available online at: https://immunisationhandbook.health.gov.au (accessed March 2025).
109. Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook: Pertussis (Whooping Cough). Canberra: Commonwealth of Australia Department of Health and Aged Care. Updated August 16, 2024. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/pertussis-whooping-cough(accessed March 2025).
110. Australian Government Department of Health and Aged Care. National Immunisation Program. Canberra: Commonwealth of Australia. Updated August 14, 2023. Available online at: https://www.health.gov.au/our-work/national-immunisation-program (accessed March 2025).
111. Australian Goverment Department of Health and Aged Care. National Immunisation Program Schedule. Canberra: Commonwealth of Australia. Updated February 4, 2025. Available online at: https://www.health.gov.au/resources/publications/national-immunisation-program-schedule?language=en (accessed March 2025).
112. Rowe SL, Leder K, Perrett KP, et al. Maternal vaccination and infant influenza and pertussis. Pediatrics 2021; 148(3): e2021051076.
113. Quinn HE, Comeau JL, Marshall HS, et al. Pertussis Disease and Antenatal Vaccine Effectiveness in Australian Children. Pediatr Infect Dis J 2022; 41: 180-185.
114. Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 2017;139(5): e20164091.
115. Australian Government Department of Health and Aged Care. Catch-up Immunisations. Camberra: Commonwealth of Australia. Updated October 15, 2024. Available online at: https://www.health.gov.au/topics/immunisation/immunisation-information-for-health-professionals/catch-up-immunisations (accessed March 2025).
116. Australian Goverment Department of Health and Aged Care. National Immunisation Catch-Up Calculator (NICC) – Cohort: Children and Adolescents Under 20 Years (v4.2). National Immunisation Program (NIP) Business Rules. Commonwealth of Australia; 2024. 64p. Available online at: https://immunisationhandbook.health.gov.au/sites/default/files/2024-06/NICC-BRS-All-people-under-20-years-v4.2.pdf (accessed March 2025).
117. Mohammed H, Roberts CT, Grzeskowiak LE, et al. Safety of maternal pertussis vaccination on pregnancy and birth outcomes: a prospective cohort study. Vaccine 2021; 39: 324-331.
118. Rowe SL, Tay EL, Franklin LJ, et al. Effectiveness of parental cocooning as a vaccination strategy to prevent pertussis infection in infants: a case-control study. Vaccine 2018; 36: 2012-2019.
119. Bayliss J, Nissen M, Prakash D, Richmond P, Oh KB, Nolan T. Control of vaccine preventable diseases in Australian infants: reviewing a decade of experience with DTPa-HBV-IPV/Hib vaccine. Hum Vaccin Immunother 2021; 17: 176-190.
120. Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr Opin Infect Dis 2010; 23: 32-38.
121. Tovo PA, de Martino M, Gabiano C, Galli L. Pertussis immunization in HIV-1-infected infants: a model to assess the effects of repeated T cell-dependent antigen administrations on HIV-1 progression. Italian Register for HIV infection in children. Vaccine 2000; 18: 1203-1209.
122. Hechter RC, Qian L, Tartof SY, et al. Vaccine safety in HIV-infected adults within the Vaccine Safety Datalink Project. Vaccine 2019; 37: 3296-3302.
123. De Vito A, Colpani A, Trunfio M, et al. Living with HIV and getting vaccinated: a narrative review. Vaccines (Basel) 2023; 11: 896.
124. Dauby N, Gagneux-Brunon A, Martin C, Mussi-Pinhata MM, Goetghebuer T. Maternal immunization in women living with HIV. AIDS 2024; 38: 137-144.
125. Nuwarda RF, Ramzan I, Weekes L, Kayser V. Vaccine hesitancy: contemporary issues and historical background. Vaccines (Basel) 2022; 10: 1595.
126. Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother 2013; 9: 1763-1773.
127. Danchin MH, Costa-Pinto J, Attwell K, et al. Vaccine decision-making begins in pregnancy: correlation between vaccine concerns, intentions and maternal vaccination with subsequent childhood vaccine uptake. Vaccine 2018; 36: 6473-6479.
128. Hutchinson AF, Smith SM. Effectiveness of strategies to increase uptake of pertussis vaccination by new parents and family caregivers: a systematic review. Midwifery 2020; 87: 102734.
129. O’Leary ST, Opel DJ, Cataldi JR, et al. Strategies for improving vaccine communication and uptake. Pediatrics 2024; 153(3): e2023065483.