Early diagnosis of COPD. Recognising the opportunities
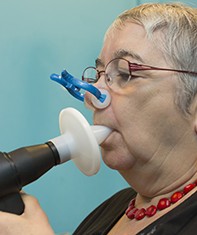
Recognising chronic obstructive pulmonary disease in primary care requires active case finding in symptomatic patients. Spirometry is essential for diagnosis.
- A diagnosis of COPD is made in a patient with typical symptoms (dyspnoea, cough, sputum) in whom spirometry demonstrates expiratory airflow obstruction that cannot be fully reversed by a bronchodilator.
- Underdiagnosis of COPD likely reflects a multitude of factors related to both patients and doctors.
- Screening of asymptomatic patients is not recommended; rather, early diagnosis in primary care relies on active case finding in symptomatic patients.
- Early diagnosis allows interventions, such as smoking cessation strategies, that may help to avoid the devastating consequences of advanced COPD.
The burden of chronic obstructive pulmonary disease (COPD) is enormous. It is currently the third leading cause of death and the fifth leading cause of disease burden globally, with disease prevalence predicted to further increase.1 It is estimated that 7.5% of people in Australia aged 40 years or over have symptomatic COPD even though half of them are undiagnosed.2 COPD accounts for almost 1% of all GP consultations, and acute exacerbations of COPD are the second leading cause of avoidable hospitalisations.3,4
A precise, accurate and satisfactory definition of COPD is surprisingly elusive. A diagnosis of COPD is made in a patient with typical symptoms (dyspnoea, cough, sputum) in whom spirometry demonstrates expiratory airflow obstruction that cannot be fully reversed by a bronchodilator. Although described as a distinct entity, in reality COPD encompasses a heterogeneous group of processes including small airways disease (obstructive bronchiolitis), chronic bronchitis and emphysema. Elements of all these processes may be present in an individual patient with COPD.
Who is at risk for COPD?
The major risk factor for COPD is cigarette smoking, although additional risk factors include occupational exposures, exposure to indoor biomass fuel burning, air pollution and childhood asthma.5-7 Symptoms of COPD may only become clinically evident after lung function has substantially declined.
In healthy people, maximal lung function is generally attained by around 20 years of age. It then plateaus until around 30 years of age before a gradual slow decline in lung function over the remainder of life. Recently, attention has increasingly focused on the multiple trajectories that can lead to low lung capacity in later life. Smoking is associated with an accelerated loss of lung function, and it is estimated that 50% of persistent smokers will ultimately fulfil criteria for COPD.8,9 Another important predisposing factor is failure to attain maximal lung capacity in earlier life.10 Although not yet fully understood, early life factors including feto-maternal health, genetics and childhood exposures may be key determinants of this failure to attain maximal lung capacity, which increases the likelihood of low lung capacity in later life.
Definition and diagnosis of COPD
A diagnosis of COPD requires spirometry demonstrating a reduced forced expiratory ratio (FER; forced expiratory volume in 1 second [FEV1] : forced vital capacity [FVC]). International guidelines define COPD as a postbronchodilator FER of less than 0.7. In essence, this means that even after bronchodilating medication is administered, of the total air forcibly expelled from the lungs after a maximal inhalation, less than 70% is expelled within the first second. When this is observed in an appropriate clinical context a diagnosis of COPD is made.
This universal threshold definition has the advantage of being simple and practical. However, because FEV1 tends to decline faster than FVC with ageing, a universal categorisation based on an FER of less than 0.7 may lead to overdiagnosis of COPD in elderly patients and underdiagnosis of COPD in younger patients. As a result, some lung function laboratories may report age-adjusted FER. It should also be noted that a degree of variability in FER measurement within individual patients can be observed, such that in patients with mild to moderate airflow limitation a single spirometry assessment may not be sufficiently reliable. For patients whose spirometry results are around the threshold level, repeat spirometry should be performed to confirm the diagnosis.
Of note, a degree of bronchodilator responsiveness is common in patients with COPD, although a response of greater than 400 mL is unusual and should prompt consideration of an asthma diagnosis. Importantly, lack of reversibility of airflow limitation after a bronchodilator does not necessarily signify lack of clinical benefit from bronchodilator therapy, as long-acting bronchodilators also significantly reduce exacerbation risk.
Spirometry is not only essential to the diagnosis of COPD, but provides a grading of COPD severity. The degree of airflow obstruction relative to a matched population reference (% predicted FEV1) has important prognostic and therapeutic relevance (Table).
Underdiagnosis and misdiagnosis of COPD
COPD is frequently underdiagnosed and misdiagnosed. It is estimated that, in the US, over 70% of patients with obstructive lung disease may be undiagnosed.11 Although many of these patients may identify few symptoms, undiagnosed COPD is associated with reduced survival.11 Conversely, it is estimated that 20 to 30% of patients given a clinical diagnosis of COPD do not demonstrate airflow obstruction on spirometry.
Although classic examination findings of diminished breath sounds and wheeze are often identified in advanced COPD, physical examination is not always reliable in mild and moderate COPD, and spirometry is essential to diagnosis.12 A study in an Australian primary care setting assessed spirometry results for patients with a clinical diagnosis of COPD and found only 58% of cases had spirometry results consistent with COPD.13
Opportunities for diagnosis of COPD are frequently missed. In a retrospective study of 38,859 patients ultimately diagnosed with COPD in UK general practice, missed opportunities for COPD diagnosis in the previous five years were identified in 85% of patients.14 By the time of COPD diagnosis, 27% were defined using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification as GOLD Stage 2 (FEV1, 50 to 79%), 15% were GOLD stage 3 (FEV1, 30 to 49%) and 10% were already GOLD stage 4 (FEV1 less than 30%). In a study of patients hospitalised for a severe exacerbation, 34% were first diagnosed with COPD at the time of hospitalisation, when they already had established severe disease and sometimes even respiratory failure.15
Barriers to diagnosis
Underdiagnosis of COPD likely reflects a multitude of factors related to both patients and doctors.
Patient factors
- COPD is a gradually progressive disease and patients may not identify the insidious onset of progressive exertional dyspnoea.
- Patients may adapt their lifestyle to avoid physical exertion that would provoke symptoms.
- Patients may attribute other key COPD symptoms such as cough and sputum production to current smoking rather than to a disease.
- Symptomatic current smokers may avoid seeking a medical diagnosis due to the stigma associated with smoking-related illness.
Doctor factors
- Doctors may attribute mild respiratory symptoms to normal ageing or effects of current smoking.
- Doctors may not challenge tobacco use.
- Perceived difficulty of access to spirometry may be a barrier to accurate diagnosis.
- GPs may have concerns regarding the accurate performance of spirometry or interpretation of results.
It is therefore important to directly ask about respiratory symptoms in patients at high risk of COPD. Respiratory symptoms in patients with a smoking history should prompt consideration of a COPD diagnosis, which can be evaluated with spirometry. Respiratory symptoms in current or ex-smokers should not be considered normal. Even when lung function is well preserved, symptomatic smokers have significantly increased rates of respiratory exacerbations.16
Handheld expiratory flow meters comparing FEV1 with FEV in 6 seconds (FEV1: FEV6) are simple to use and have shown utility as a screening test for identifying people most likely to benefit from a formal spirometry assessment for COPD (FEV1: FVC).17 Training courses for office spirometry in primary care are available (https://www.nationalasthma.org.au/health-professionals/education-training/spirometry-training). Referral to a pulmonary function laboratory is otherwise required for spirometry.
Should population screening be performed to identify COPD at its earliest stage?
Given the progressive natural history and devastating impact of advanced COPD, it is logical to consider a role for screening to facilitate diagnosis at an early stage of disease. Screening for asymptomatic COPD using questionnaires and simple prebronchodilator spirometry devices has been investigated but is not currently routinely recommended.18 It is important to consider the difference between screening apparently healthy people for occult disease and case finding, which is the targeted evaluation of high-risk groups to make a diagnosis earlier than would occur by waiting for them to present with symptoms or signs.
The key interventions in COPD management are aimed at alleviation of symptoms and prevention of exacerbations. Patients with very mild COPD may truly be asymptomatic and such patients are generally at relatively low levels of exacerbation risk. As such, population screening of asymptomatic individuals is not indicated for COPD given insufficient evidence of benefit.19 In contrast, active case finding of COPD in symptomatic patients in primary care is recommended.20 In this regard, given the tendency of patients with mild COPD to underreport respiratory symptoms, vigilance from their GP is highly valuable.
Case finding for COPD
Active case finding for COPD appears to be more effective than opportunistic case finding. A randomised controlled trial among primary care patients in the UK compared active case finding that identified patients with COPD using a mailed questionnaire with opportunistic case finding using a questionnaire at the time of GP consultation. Active case finding identified more cases that were subsequently confirmed with spirometry (odds ratio, 2.34) and also appeared more cost-effective.21,22
What difference would early diagnosis and intervention make?
Earlier diagnosis and introduction of evidence-based interventions reduce morbidity and mortality in patients with COPD. The steps involved in early diagnosis and treatment of patients with COPD are summarised in the Box.
Smoking cessation
Smoking cessation is the most important intervention in COPD management. The accelerated decline in lung function associated with smoking is significantly reduced after quitting.23 It is recommended that clinicians ask all adult patients about smoking and offer smoking cessation interventions to current smokers.24 Evidence supports the impact of smoking cessation interventions from simple clinician advice to pharmacotherapy;25,26 however, most studies to date have not shown that evidence of COPD from spirometry increases smoking cessation rates.27 Of note, expressing lung function impairment to patients using the terminology of ‘lung age’ relative to their chronological age may have more impact on smoking cessation rates.28,29 Further research into optimising smoking cessation strategies in this context is required.
Exercise and pulmonary rehabilitation
Regular physical activity is of key importance in patients with COPD. It can maintain function and is associated with reduced hospitalisation rates and mortality.30 Pulmonary rehabilitation is a tailored exercise and education program that has been shown to improve symptoms and reduce exacerbations in patients with COPD.31 Local centres offering pulmonary rehabilitation can be found on the Lung Foundation Australia website (https://lung foundation.com.au/exercise-classes). Patients can be referred directly from primary care.
Vaccination
Vaccination for influenza is recommended annually for patients with COPD. Pneumococcal vaccination is also recommended.
Pharmacotherapy
In reality, at the time of initial diagnosis many patients with COPD already have established disease with respiratory symptoms and often a history of exacerbations. Pharmacotherapy with long-acting bronchodilators and, for some patients, inhaled corticosteroids will often be indicated. Concise guidelines for comprehensive management of COPD in primary care are available (https://copdx.org.au).
Comorbidities
COPD is frequently associated with comorbidities including cardiovascular disease, anxiety, depression and osteoporosis. High vigilance in primary care for coexisting cardiac disease is of key importance. Spirometric evaluation of patients with suspected COPD is one of the steps required to disentangle the respiratory and cardiac contributions in the breathless patient. Cardiac disease is highly prevalent but underdiagnosed and undertreated in COPD populations. Evaluation of cardiac health and cardiac risk factors in primary care is recommended.32
Conclusion
COPD is highly prevalent but underdiagnosed. Opportunities for earlier diagnosis of COPD are frequently missed and patients often have advanced disease by the time of diagnosis. Spirometry is the key investigation for diagnosing COPD. RMT