Frailty and chronic lung disease: a silent overlap in need of attention

Frailty is a syndrome that may be considered common in older patients; however, its presence and impact among younger individuals living with chronic lung disease is often underappreciated. As risk factors vary between patients, assessment and management require an individualised approach with input from a multidisciplinary team.
- Frailty is an important but complex medical syndrome associated with increased susceptibility to negative health consequences or adverse events. It is not a normal part of healthy ageing and is common in people with chronic lung disease.
- Because the risk factors for frailty and chronic lung disease overlap, the impact on younger patients is often overlooked.
- Frailty screening can be performed using various tools, with local circumstances and preferences often dictating choice.
- High-quality, personalised management of frailty in people with chronic lung disease necessitates a thorough assessment to identify specific causative factors and individual risks.
- Considering its multidimensional genesis, best practice comprehensive management of frailty typically involves the co-ordinated interplay of various healthcare professionals.
- Simple actions to assist in managing people with chronic lung disease who have frailty include early referral to local pulmonary rehabilitation programs, dietitians and geriatricians.
‘If a tree falls in a forest and no one is around to hear it, does it make a sound?’
This saying is relevant to the care of people with chronic lung disease, as identifying clinically important events as markers of the disease versus their associated complications is challenging.
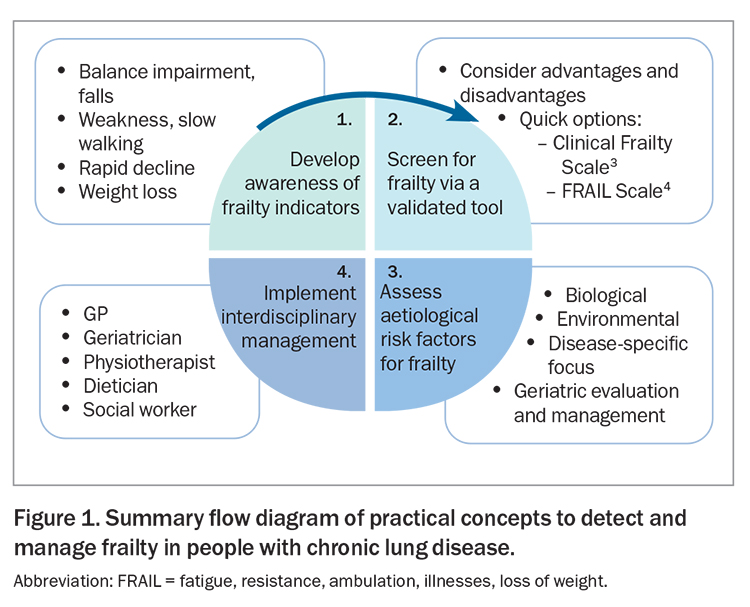
People with chronic lung disease, such as chronic obstructive pulmonary disease (COPD), experience functional limitations, poorer quality of life and increased health care dependency compared with average ‘healthy’ community-dwelling adults. People who have frailty are susceptible to exaggerated consequences of health insults and have an increased risk of adverse events, such as falls, institutionalisation and health care dependency. Acute respiratory exacerbations (regardless of whether they result in hospitalisation) are important occurrences that exert a range of negative health impacts on individuals. They are clinically heterogeneous events, and often accepted as part of the progressive nature of chronic lung disease (strong ‘noise-to-signal’ ratio).1 Rarely, however, do we consider the extent to which the presence of frailty may have impacted such events. Yet, the overlapping coexistence of chronic lung disease and frailty is common and exerts a compounding form of intersectionality.2 Failure to look for frailty confines it to the ‘falling tree in the woods’. This article discusses the options available to comprehensively manage frailty comprehensively in people with chronic lung disease. Practical concepts to detect and manage frailty in people with chronic lung disease are presented in Figure 1.3,4
Frailty in chronic lung disease
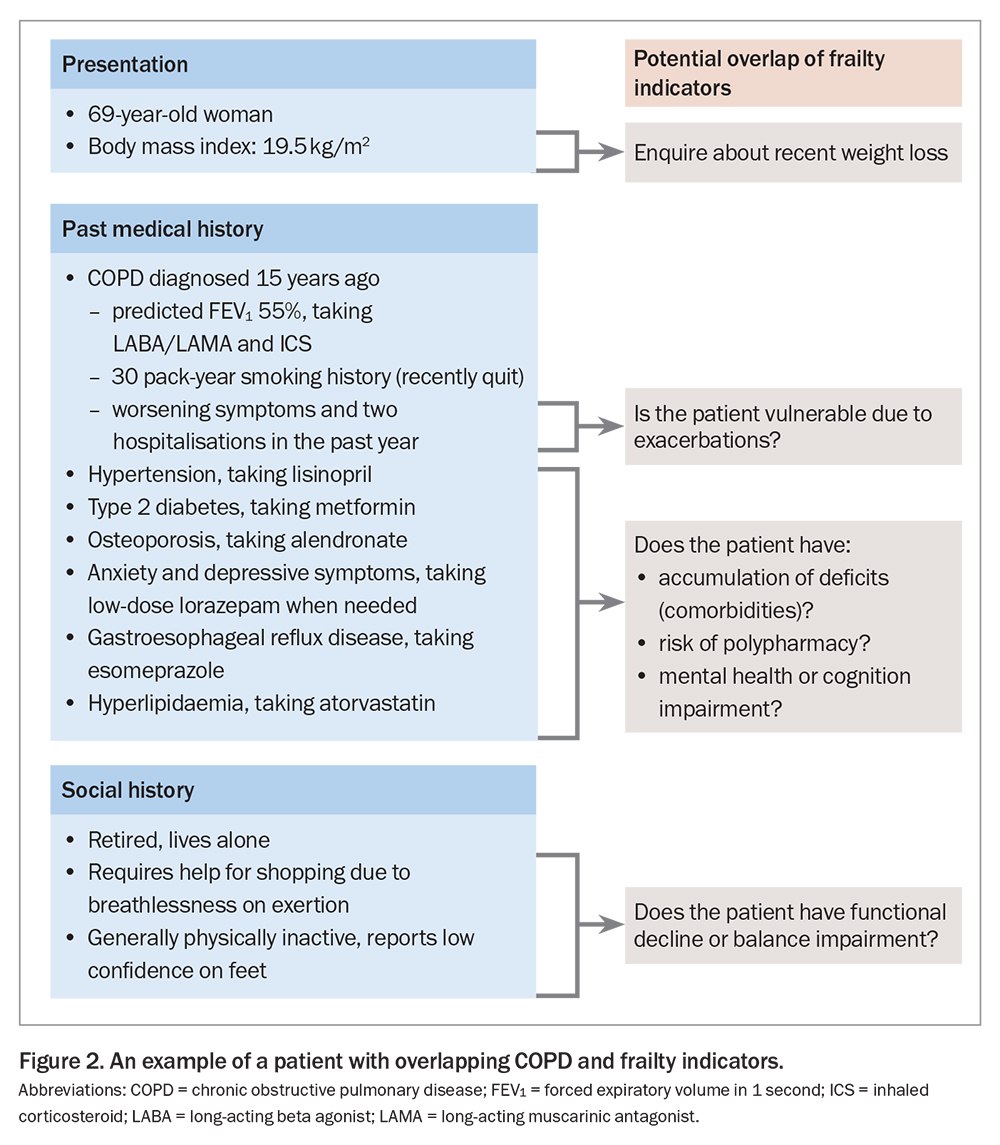
Uncertainty has existed for some time regarding the precise impact and role of frailty in the context of chronic lung disease. Emerging precision medicine models of lung health care, such as the ‘treatable traits’ paradigm, have shed an overdue spotlight on frailty in recent years.5 Confusion regarding frailty has partly stemmed from the many ways it has been defined (a generic condition vs specific forms), measured (objectively vs subjectively) and discussed (lay vs scientific terminology). An international expert European Respiratory Society Task Force recently addressed a comprehensive range of issues related to frailty, advocating for frailty to be defined in the context of chronic lung disease as it is in geriatric medicine, as a multidimensional syndrome characterised by decreased physiological reserves and diminished resistance to stressors.6,7 This generalised definition is helpful as it applies equally across many diseases. The causes of frailty, however, are typically driven by a complex interplay of factors that can have disease-specific origins. For example, risk factors for developing COPD, including biophysiological and environmental exposures and ranging from preterm and early life to the middle and older years of life, may also predispose to the development of features of frailty, such as poor muscle function, physical inactivity, malnutrition, impaired mental health and polypharmacy (including side effects of oral corticosteroid use).8 This overlap in risk factors means frailty is not restricted to people of older age. Acute respiratory exacerbations worsen many such factors, and individuals are particularly prone to a heightened negative impact of frailty during these periods. A case example demonstrating these overlapping features is presented in Figure 2.
How to assess for frailty in clinical practice
Various screening instruments exist to aid in the detection of frailty in clinical practice, with no single tool considered a ‘gold standard’. Benefits (e.g. ease and quick time to administer) must be weighed against inherent costs (e.g. expertise required to administer, comprehensiveness of the tool), with the choice of tool ultimately guided by local needs and purpose of use. In general, tools align with one of two accepted methods for conceptualising frailty.
- The accumulated deficits approach (or the ‘Rockwood’ model) is premised on the basis that as people age and acquire more health problems, they are more likely to develop frailty.9 Tools aligned with this approach tend to evaluate the presence (yes/no) of a range of different comorbid conditions, with a score often calculated to indicate a ratio of the number of items detected against those assessed.
- The phenotype (or ‘Fried’) model is premised on frailty having a predictable negative impact on an individual’s physical condition.10 Tools aligned to the phenotype model encompass various methods to evaluate five core areas: weight loss, exhaustion, physical activity, muscle function and mobility.
Routine screening for frailty is not presently indicated for people with chronic lung disease; however, options exist to optimise its timely detection. For example, the electronic Frailty Index is a validated assessment tool based on the Rockwood model of frailty that evaluates 36 deficits spanning various clinical signs and symptoms, comorbidities and test results.11 It is capable of being assessed from existing medical health records and can be integrated within primary care systems to optimise data collection efficiencies. Several studies describe how this tool can be adapted to suit local data capture methods, including its implementation in Australian rural health settings.12,13
Two other commonly used validated tools for assessing frailty are the Clinical Frailty Scale (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7458601/) and the FRAIL (fatigue, resistance, ambulation, illnesses, loss of weight) Scale (https://sydneynorthhealthnetwork.org.au/wp-content/uploads/2018/12/PDF-Frail-scale.pdf).3,4
Another important factor to help optimise the detection of frailty is to refine our awareness of ‘frailty indicators’. Examples of frailty indicators include slow walking speed, balance impairments or reports of falls, confusion, hospitalisations, unexpected weight loss or exaggerated declines in function from relatively minor insults (e.g. from a mild virus). The detection of such issues, even in the presence of plausible identifiable causes, should lead to a more formal and timely evaluation of frailty. Importantly, evaluation does not stop at assessment via a frailty screening tool; screening results should trigger a comprehensive evaluation process to better understand the likely aetiology of frailty so that management strategies can be personalised to an individual’s needs. In Australia, much of this management is co-ordinated by the GP in primary care; however, it can be valuable to source expert geriatrician input when appropriate. Geriatricians play a key part in the assessment and management of frailty in many countries, and are central to the best practice recommended care process called ‘Comprehensive Geriatric Assessment’ (sometimes referred to as ‘Geriatric Evaluation and Management’ or GEM in Australia). Further information on Comprehensive Geriatric Assessment is available online at: https://www.bgs.org.uk/cgatoolkit.
Managing frailty in chronic lung disease
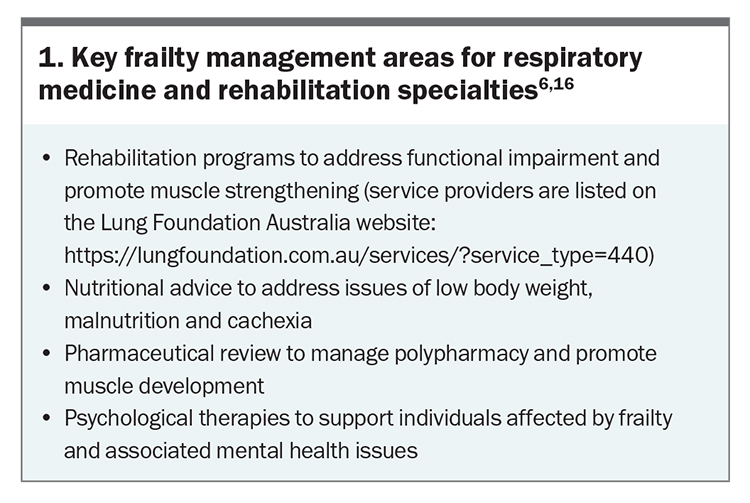
Disease-specific evidence to help clinicians manage frailty in people with chronic lung disease is relatively scarce, due in part to frailty being a common criteria for exclusion from many clinical trials. Australia and New Zealand’s COPD-X guideline offers some of the most detailed guidance of the international literature (Section 07.4 – Frailty in COPD).6,14 General information about frailty in older people is more widely available from resources such as the RACGP ‘Silver Book’15 and online summaries (https://www.cgakit.com/frailty). Comprehensive insights into frailty management in the context of respiratory medicine for rehabilitation have been recently published.6,16 Key management areas covered in these reports are summarised in Box 1. In order to optimise outcomes for people with chronic lung disease who have frailty, it is essential that GPs accurately recognise frailty as a dynamic syndrome (i.e. one that can improve or worsen over time) that is treatable via well-co-ordinated, comprehensive management. Serial assessments are essential to monitor responses to therapies and changes in frailty status and severity over time, and close monitoring of the frequency and impact of acute respiratory exacerbations is particularly important as disease progresses. An overview of key messages for GPs to help guide this management process is presented in Box 2.
Conclusion
Frailty is a broadly applicable but very individual syndrome that has both common and unique impacts on patients, their families and carers and the healthcare system. Clinicians can enhance the early detection and comprehensive management of frailty through vigilant awareness of frailty indicators and collaborative multidisciplinary input. RMT
COMPETING INTERESTS: None.
References
1. MacDonald MI, Osadnik CR, Bulfin L, et al. MULTI-PHACET: multidimensional clinical phenotyping of hospitalised acute COPD exacerbations. ERJ Open Res 2021; 7: 00198-2021.
2. Marengoni A, Vetrano DL, Manes-Gravina E, et al. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest 2018; 154: 21-40.
3. Rockwood K, Theou O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J 2020; 23: 210-215.
4. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16: 601-608.
5. McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur Respir J 2019; 53: 1802058.
6. Osadnik CR, Brighton LJ, Burtin C, et al. European Respiratory Society statement on frailty in adults with chronic lung disease. Eur Respir J 2023; 62: 2300442.
7. Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14: 392-397.
8. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet 2022; 400: 921-972.
9. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1: 323-336.
10. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146-M156.
11. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age and Ageing 2016; 45: 353-360.
12. Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24.
13. Ambagtsheer RC, Beilby J, Dabravolskaj J, et al. Application of an electronic Frailty Index in Australian primary care: data quality and feasibility assessment. Aging Clin Exp Res 2019; 31: 653-660.
14. Yang IA, George J, McDonald CF, et al. The COPD-X Plan: Australian and New Zealand Guidelines for the management of chronic obstructive pulmonary disease 2022. Version 2.68, October 2022.
15. The Royal Australian College of General Practitioners (RACGP). Part A - Frailty. 2019. In: RACGP aged care clinical guide (Silver Book) 5th ed. East Melbourne, Vic: RACGP, 2019, 2020.
16. Maddocks M, Brighton L, Alison J, et al. Rehabilitation for people with respiratory disease and frailty: an official American Thoracic Society workshop report. Ann Am Thorac Soc 2023; 20: 767-780.