Preterm lung disease: not just for neonatologists
Improvements in neonatal critical care have resulted in more people than ever reaching adulthood after being born prematurely. At the same time, it is becoming clearer that preterm birth can increase the risk of respiratory disease throughout a person’s lifetime. Awareness that a patient was born preterm can enable early specialist assessment and intervention when there is any concern about lung health.
- Earlier improvements in neonatal critical care mean that more survivors of preterm birth are now reaching adulthood.
- Preterm birth is associated with increased respiratory symptoms and lung function deficits throughout the life course.
- Lung health trajectories suggest that, without intervention, many adult survivors of preterm birth will develop early-onset chronic obstructive pulmonary disease.
- More research is needed to optimise treatment guidelines for preterm lung disease.
- Individuals presenting to primary care should be asked if they were born preterm and, if so, referred for specialist pulmonary function testing.
When was the last time you asked if your patient was born prematurely?
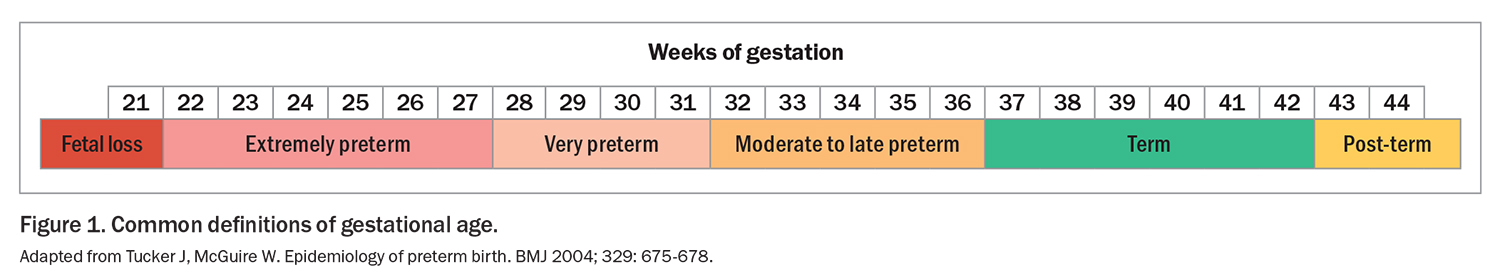
Preterm birth, defined as birth before 37 weeks’ completed gestation, affects about 15 million babies around the world every year.1 Preterm births can be further subcategorised as very preterm (before 32 weeks’ gestation) and extremely preterm (before 28 weeks’ gestation), with the lower limit of viability at about 22 weeks’ gestation (Figure 1).2 In Australia, almost 25,000 babies were born preterm in 2020, accounting for 8.3% of all live births.3 Nearly 5000 of these were born very preterm.3 Preterm birth rates are even higher in Indigenous Australians (14% of births).4
Rapid improvements in neonatal critical care during the 1990s, such as surfactant replacement therapy to reduce respiratory morbidity, have drastically increased survival rates of preterm infants.5 Today, almost 90% of babies born very preterm survive until discharge if they receive intensive care.6 These increased odds of survival are resulting in more survivors of preterm birth and low birthweight reaching adulthood than at any other time in history.7 As this first generation of people to benefit from neonatal lifesaving technologies start approaching middle age, we need to be more aware of the respiratory consequences of preterm birth that continue throughout life. In this article, we outline the evidence that shows prematurity does not just cause respiratory problems in infancy and highlight why preterm birth should be considered a key part of medical history.
Respiratory consequences of preterm birth
Early life in the NICU
The course that preterm infants follow in the neonatal intensive care unit (NICU) varies greatly between infants because of multiple incompletely understood factors. However, most infants born very or extremely preterm will require respiratory support in their initial days. The duration and type of respiratory support required will influence whether a baby is diagnosed with the chronic lung disease of prematurity, termed bronchopulmonary dysplasia (BPD). Although several definitions of BPD have been proposed over the years, there is no set diagnostic test to determine if an infant has BPD. Rather, a label of BPD has typically been applied if respiratory support is still required by 36 weeks’ postmenstrual age, or if more than 28 days of supplemental oxygen are required for those born before 32 weeks’ gestation.8
Attention should also be paid to the risk of pulmonary hypertension developing in those born preterm. The incidence of pulmonary hypertension in premature infants is suggested to be about 20%, with the greatest risk seen in children with BPD.9,10
Infancy and early life
Some infants will have a persistent supplemental oxygen requirement and may be discharged from the NICU with home oxygen therapy or, in rare cases in Australia, with greater levels of respiratory support, including ventilation via tracheostomy. Children requiring ongoing respiratory support after being discharged from the NICU will typically be followed up by respiratory specialists in a tertiary respiratory centre during the acute (active treatment) phase. There are no recommendations regarding best practice for follow up of these children or how best to wean respiratory support, so practices tend to be dependent on the experience of the treating centre.11 An infant with a neonatal BPD diagnosis and home oxygen use is considered at high risk of ongoing respiratory complications; however, this should not be used as the only predictor of ongoing morbidity. There is currently no co-ordinated respiratory follow up of infants discharged home from the NICU without a requirement for ongoing respiratory support.
It should be emphasised that children born preterm typically do not ‘catch up’ with respect to their lung function in the first years of life, and they may fall further below predicted values.12 Risk of hospital readmission is high during early life, with about one in four preterm infants requiring readmission in their first year.13 This partially relates to the multifaceted deficiencies in their relatively immature immune systems, which increases their susceptibility to infection.14 Indeed, the leading cause for readmission is viral infection, with about 50% of infants diagnosed with BPD having an infection-related hospital admission before the age of 3 years.15 Palivizumab is consequently recommended for at-risk infants when respiratory syncytial virus is circulating in the community.16 However, the associated costs mean that eligibility criteria and implementation of this intervention vary across Australian centres.17,18 Early-life hospitalisations for severe respiratory viral infection are associated with lower lung function in young adulthood and should be considered a red flag for closer monitoring.19
Although respiratory infection is a major driver of early-life wheeze, the risk of persistent wheeze is elevated in those born prematurely and shows an inverse relationship with gestational age.20 Higher rates of airway malacia, defined as increased airway compliance or dynamic airway collapse, have been reported in preterm infants.21 This disease may contribute to symptoms such as wheeze and necessitate additional respiratory support and treatment.21,22 Sleep disordered breathing is also more prevalent in those born preterm, with increased rates of obstructive and central sleep apnoea.23,24 In children with a history of airway trauma, such as from early-life intubation, physicians should remain vigilant for airway stridor, which may indicate the presence of subglottic stenosis or airway cysts.25,26
Childhood and adolescence
With increasing age, the risk of respiratory-related hospitalisation for preterm-born children decreases, although it remains higher than in the term-born population.27 Many children will be asymptomatic from a respiratory perspective. This likely reflects the natural increase in airway diameter and lung function that occurs during childhood growth, with peak lung function typically achieved in late adolescence or early adulthood. However, healthcare usage for those born preterm remains higher than for the general population until at least 10 years of age.28 It is not uncommon for children to report ongoing respiratory symptoms, including cough and wheeze, particularly in conjunction with exercise or acute respiratory illness.29-32 Cohort studies assessing pulmonary function during childhood show reduced function in those born preterm compared with term-born counterparts, with spirometry typically showing an obstructive pattern.30,33-35 Cohort studies and a meta-analysis show a bronchodilator response is present in about 20 to 30% of preterm children, although rates can be significantly higher in those with BPD.30,35,36 The presence of a bronchodilator response aligns with the higher asthma risk observed in this population.37,38 However, it is likely that the mechanisms underpinning symptoms in preterm children differ from those driving typical childhood asthma. For example, eosinophilic inflammation is far less common in those born preterm than in those born at term or in children with asthma.39
Although respiratory symptoms are typically mild during childhood, several cohort studies have found that lung function trajectories in those born preterm track further from those of the normal-term population as they move through adolescence.33,35 Additionally, a recent meta-analysis pooling data from 1326 preterm individuals shows evidence of increasing airway obstruction with age after a diagnosis of BPD as an infant.40 If such decline continues on top of already reduced lung function, it is likely that respiratory symptoms will worsen with increasing age. Of concern, a comparative cohort study suggests lung function in preterm-born children has not improved over recent decades, despite significant advances in neonatal ventilation techniques.35 A further important finding from longitudinal studies is that respiratory impairment is not confined to children who were born very preterm or who required supplemental oxygen; children born moderate to late preterm can also have airway obstruction when measured by spirometry.41,42
Although pulmonary hypertension is often detected in the NICU, it may arise in childhood or later in life. Monitoring with echocardiography should be used if there is any concern about pulmonary hypertension.43
Adulthood
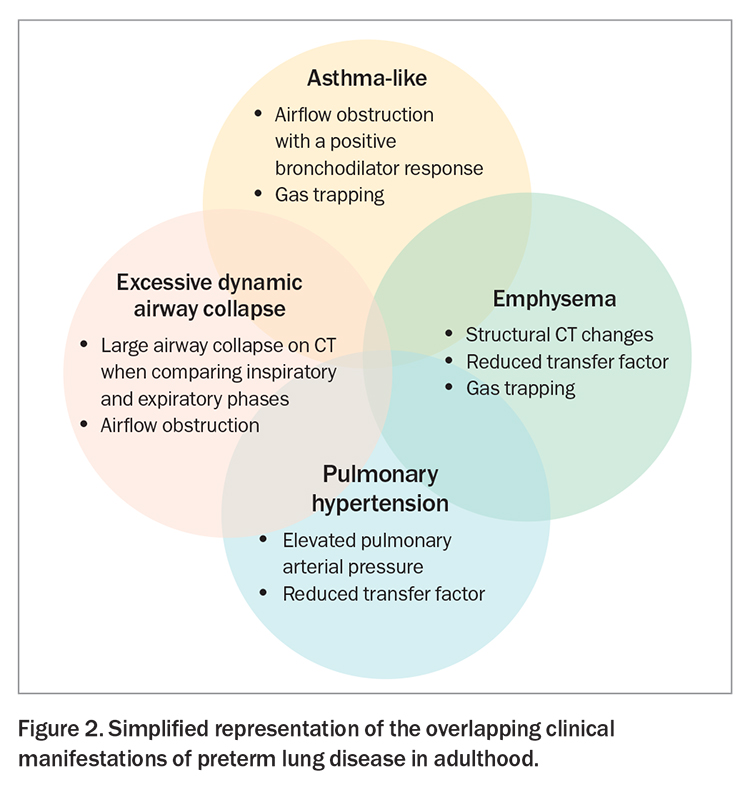
The natural limitation that many survivors of preterm birth are only now entering middle age has restricted our knowledge about the consequences of preterm birth in adulthood. It is clear from multiple cohort studies, however, that adults who were born preterm are more likely to report respiratory symptoms, have persisting deficits in lung function test results and have radiological abnormalities.44 The overlap of presenting symptoms with other respiratory conditions can confuse diagnosis, and a history of preterm birth may be overlooked (Figure 2).
There is growing concern that some survivors of extremely to moderate preterm birth are on a trajectory to develop early-onset chronic obstructive pulmonary disease (COPD) or respiratory failure. A recent analysis from the Tasmanian Longitudinal Health Study focusing primarily on people born moderate to late preterm showed an increased risk of COPD by the age of 53 years.45 This builds on previous work showing emphysematous changes on CT scans of young adults who had survived preterm birth.46 A Lancet Commission highlighted prematurity as a significant risk factor for COPD, and the 2023 Global Initiative for Chronic Obstructive Lung Disease guidelines propose ‘COPD due to abnormal lung development (COPD-D)’ as a new classification of COPD with origins in neonatal and early life.47,48
Alarmingly, case reports of lung transplants being needed in those with severe lung disease after preterm birth are starting to emerge.49,50 The median age of transplantation was found to be just 21 years in patients with a history of BPD (compared with a median age of 57 years for those born at term), with the incidence increasing steadily since the early 2000s.49 Although these data have primarily been collected from patients born in the presurfactant therapy era, progressive airway obstruction is similarly described in young adult survivors of the postsurfactant therapy era.51
How do we treat preterm lung disease?
Preventing preterm birth
Although preventing preterm birth is the most effective way to stop future cases of preterm lung disease, this does not help the existing population of preterm birth survivors and, to date, has been an unrealistic goal. Rates of preterm birth have remained largely unchanged over recent decades.52 Similarly, rates of BPD have remained consistent, despite advances in neonatal care.53 Preventing preterm birth remains an important goal but is unlikely to be a solution to preterm lung disease in coming years.
Identifying a history of preterm birth
The high incidence of survivors of preterm birth in the community highlights that all healthcare providers, from GPs through to tertiary clinicians, are likely to treat someone who was born preterm throughout their career. However, to adequately treat the respiratory sequelae experienced by survivors of preterm birth, clinicians must first identify that an individual was born preterm. Notably, 80% of participants in a recent survey of adults born preterm stated that their healthcare providers did not ask about their history of prematurity, nor did they consider it when recommending treatment options.54 A survey of respiratory physicians in the United Kingdom found that only 20% routinely ask if their patient was born preterm, with paediatric specialists most likely to ask the question.55 This unawareness of relevant medical history creates the risk of thousands of individuals being misdiagnosed or mistreated for conditions they do not have, such as asthma.
Although not the focus of this article, asking about prematurity may also provide insights into increased risks of the early onset of chronic diseases outside the respiratory system, such as glucose intolerance, osteopenia, hypertension and cardiac dysfunction. When combined with lung disease, these result in a 40% increase in the risk of premature death in preterm-born young adults.56 A lack of clinician knowledge and education about the long-term consequences of preterm birth has been suggested as the main barrier preventing collection of early-life history, highlighting the need for increased awareness.57,58 The recently launched Lung Learning Framework (www.lunglearninghub.com.au), which provides a competency-based framework for diagnosing and supporting complex lung conditions, may be a useful starting point for healthcare professionals who wish to improve their knowledge.
Investigation
When there is a concern about impaired lung health in someone who was born preterm, further investigation is needed to identify the type and severity of the impairment. Near normal spirometry in primary care should not be taken as reassurance of only a minor problem, and specialist referral should be considered at an early stage to understand the phenotype present.59 This assessment may include full lung function testing, an echocardiogram and a chest CT scan. In those with suspected small airways disease, especially with comorbid large airway collapse, oscillometry can be a useful tool. Cardiopulmonary exercise testing may also be used to better understand the degree of impairment of various aspects of the cardiopulmonary system.
Treatments and intervention
Clinical care of those born preterm is complicated by a lack of clear treatment guidelines. The latest position statement from the Thoracic Society of Australia and New Zealand on managing neonatal chronic lung disease beyond the intensive care unit focuses on the early-life period, offering no advice for older patients.16 The European Respiratory Society guideline on long-term management of BPD notes that its recommendations are based on low-quality evidence but suggests that bronchodilators only be prescribed if reversibility in lung function or asthma-like symptoms are observed, with a blanket recommendation against prescribing inhaled corticosteroids.60 However, conflicting evidence from recent clinical trials suggests that inhaled corticosteroid treatment may benefit some preterm-born individuals, particularly those with a bronchodilator response.61,62
Ideally, individuals should receive a systematic multidisciplinary assessment to ensure all treatable aspects of their condition are dealt with. This includes identification and management of cardiorespiratory abnormalities, such as airflow obstruction and pulmonary hypertension, but also preventive care, such as vaccination, smoking cessation, optimisation of function through exercise physiology or physiotherapy and psychological support. As many people who were born preterm also have neurodevelopmental problems, additional time and effort may be needed to ensure they have good disease understanding and adherence to therapy.
Where do we go from here?
We still have a long way to go in understanding the full picture of preterm lung disease. As survivors from the postsurfactant therapy era get older, respiratory health consequences are continuing to emerge. Simultaneously, new generations are being born with their own unique exposures and treatments that were not available to previous generations. We do not yet know what mechanisms are driving ongoing disease and lung function decline in older survivors or how the landscape of preterm lung disease might change with younger generations. Continued research in this population group will be essential for optimal care and treatment. Cohort studies that continue throughout adolescence and into adulthood, such as the Western Australian Lung Health in Prematurity cohort and the Victorian Infant Collaborative Study, will provide much needed insight into disease progression throughout the life course.33,35,51
Clinical trials are also necessary to establish whether current treatment options are efficacious in those who were born preterm. Basic science and laboratory studies will be essential to understand the mechanisms driving ongoing disease, which will then allow for the development of more targeted treatment options. Not to be overlooked is the importance of identifying modifiable exposures and risk factors in early life, as well as ways to identify which individuals are most at risk of ongoing morbidity. The results of such studies will be crucial for the clinical management of this expanding population.
Underpinning these future needs is the core matter of how Australian health care is organised; there is currently no robust network of specialist centres that can co-ordinate and advance clinical care, research and education in this area. Respiratory follow up or screening are not routine in this population and typically only occur if an individual is enrolled in a research program or reaches a point where symptoms warrant further investigation.
Conclusion
Despite the striking evidence that respiratory sequelae of preterm birth continue throughout the life course, it appears that child, adolescent and adult survivors of preterm birth are getting lost in the transitions from neonatal to paediatric to adult health care. A pressing priority is ensuring that all physicians recognise the growing burden and importance of chronic respiratory disease in this population. We encourage all healthcare providers to start asking their patients: were you born preterm? RMT
COMPETING INTERESTS: None.
References
1. March of Dimes, The Partnership for Maternal, Newborn & Child Health, Save the Children, World Health Organization. Born too soon: the global action report on preterm birth. Geneva: WHO; 2012.
2. Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004; 329: 675-678.
3. Australian Institute of Health and Welfare. Australia’s mothers and babies. Canberra: AIHW; 2022.
4. Australian Institute of Health and Welfare. Pregnancy and birth outcomes for Aboriginal and Torres Strait Islander women: 2016–2018. Canberra: AIHW; 2021.
5. Gultom E, Doyle LW, Davis P, Dharmalingam A, Bowman E. Changes over time in attitudes to treatment and survival rates for extremely preterm infants (23-27 weeks’ gestational age). Aust N Z J Obstet Gynaecol 1997; 37: 56-58.
6. Cheong JLY, Olsen JE, Huang L, et al. Changing consumption of resources for respiratory support and short-term outcomes in four consecutive geographical cohorts of infants born extremely preterm over 25 years since the early 1990s. BMJ Open 2020; 10: e037507.
7. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379: 2162-2172.
8. Ibrahim J, Bhandari V. The definition of bronchopulmonary dysplasia: an evolving dilemma. Pediatr Res 2018; 84: 586-588.
9. Lagatta JM, Hysinger EB, Zaniletti I, et al. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J Pediatr 2018; 203: 218-224.e3.
10. Kim YJ, Shin SH, Park HW, Kim E-K, Kim H-S. Risk factors of early pulmonary hypertension and its clinical outcomes in preterm infants: a systematic review and meta-analysis. Sci Rep 2022; 12: 14186.
11. Everitt LH, Awoseyila A, Bhatt JM, Johnson MJ, Vollmer B, Evans HJ. Weaning oxygen in infants with bronchopulmonary dysplasia. Paediatr Respir Rev 2021; 39: 82-89.
12. Hofhuis W, Huysman MWA, van der Wiel EC, et al. Worsening of V′maxFRC in infants with chronic lung disease in the first year of life. Am J Respir Crit Care Med 2002; 166: 1539-1543.
13. Pramana IA, Latzin P, Schlapbach LJ, et al. Respiratory symptoms in preterm infants: burden of disease in the first year of life. Eur J Med Res 2011; 16: 223-230.
14. Collins A, Weitkamp J-H, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed 2018; 103: F391-F394.
15. Hong T, Bolisetty S, Bajuk B, et al. A population study of respiratory rehospitalisation in very preterm infants in the first 3 years of life. J Paediatr Child Health 2016; 52: 715-721.
16. Kapur N, Nixon G, Robinson P, et al. Respiratory management of infants with chronic neonatal lung disease beyond the NICU: a position statement from the Thoracic Society of Australia and New Zealand. Respirology 2020; 25: 880-888.
17. Trist S, Horsley E, Katf H, Tasker N, Mostaghim M. Improving the prescribing of palivizumab. J Paediatr Child Health 2018; 54: 1353-1356.
18. Xu R, Fathima P, Strunk T, et al. RSV prophylaxis use in high-risk infants in Western Australia, 2002-2013: a record linkage cohort study. BMC Pediatr 2020; 20: 490.
19. Smith EF, Hemy NR, Hall GL, Wilson AC, Murray CP, Simpson SJ. Risk factors for poorer respiratory outcomes in adolescents and young adults born preterm. Thorax 2023; May 19. doi: 10.1136/thorax-2022-219634. Online ahead of print.
20. Been JV, Lugtenberg MJ, Smets E, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001596.
21. Wang T, Xu Q, Dai G, et al. Clinical characteristics of children with airway malacia complicated by pneumonia. BMC Infect Dis 2021; 21: 902.
22. Wallis C, Alexopoulou E, Antón-Pacheco JL, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J 2019; 54: 1900382.
23. Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep 2012; 35: 1475-1480.
24. Sharma PB, Baroody F, Gozal D, Lester LA. Obstructive sleep apnea in the formerly preterm infant: an overlooked diagnosis. Front Neurol 2011; 2: 73.
25. See GB, Mesran I. Stridor secondary to acquired subglottic cyst: rarity makes it missed. Indian J Otolaryngol Head Neck Surg 2019; 71 Suppl 1: 45-48.
26. Nolder AR, Richter GT. The infant with noisy breathing. Curr Treat Options Pediatr 2015; 1: 224-233.
27. Stevenson PG, Cooper MN, Billingham W, et al. Health service utilisation for acute respiratory infections in infants graduating from the neonatal intensive care unit: a population-based cohort study. BMC Pediatr 2023; 23: 335.
28. Westrupp EM, Lucas N, Mensah FK, Gold L, Wake M, Nicholson JM. Community-based healthcare costs for children born low birthweight, preterm and/or small for gestational age: data from the Longitudinal Study of Australian Children. Child Care Health Dev 2014; 40: 259-266.
29. Kotecha SJ, Watkins WJ, Lowe J, Granell R, Henderson AJ, Kotecha S. Comparison of the associations of early-life factors on wheezing phenotypes in preterm-born children and term-born children. Am J Epidemiol 2019; 188: 527-536.
30. Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 2010; 182: 237-245.
31. Vrijlandt EJLE, Kerstjens JM, Duiverman EJ, Bos AF, Reijneveld SA. Moderately preterm children have more respiratory problems during their first 5 years of life than children born full term. Am J Respir Crit Care Med 2013; 187: 1234-1240.
32. Verheggen M, Wilson AC, Pillow JJ, Stick SM, Hall GL. Respiratory function and symptoms in young preterm children in the contemporary era. Pediatr Pulmonol 2016; 51: 1347-1355.
33. Simpson S, Turkovic L, Wilson A, et al. Lung function trajectories throughout childhood in survivors of very preterm birth: a longitudinal cohort study. Lancet Child Adolesc Health 2018; 2: 350-359.
34. Simpson SJ, Logie KM, O’Dea CA, et al. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 2017; 72: 702-711.
35. Doyle LW, Adams AM, Robertson C, et al. Increasing airway obstruction from 8 to 18 years in extremely preterm/low-birthweight survivors born in the surfactant era. Thorax 2017; 72: 712-719.
36. Kotecha SJ, Edwards MO, Watkins WJ, Lowe J, Henderson AJ, Kotecha S. Effect of bronchodilators on forced expiratory volume in 1 s in preterm-born participants aged 5 and over: a systematic review. Neonatology 2015; 107: 231-240.
37. Jaakkola JJK, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol 2006; 118: 823-830.
38. Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics 2011; 127: e913-e920.
39. Course CW, Kotecha S, Kotecha SJ. Fractional exhaled nitric oxide in preterm-born subjects: a systematic review and meta-analysis. Pediatr Pulmonol 2019; 54: 595-601.
40. Gibbons JTD, Course CW, Evans EE, Kotecha S, Kotecha SJ, Simpson SJ. Increasing airway obstruction through life following bronchopulmonary dysplasia: a meta-analysis. ERJ Open Res 2023; 9: 00046-2023.
41. Kotecha SJ, Watkins WJ, Paranjothy S, Dunstan FD, Henderson AJ, Kotecha S. Effect of late preterm birth on longitudinal lung spirometry in school age children and adolescents. Thorax 2012; 67: 54-61.
42. Du Berry C, Nesci C, Cheong JLY, et al. Long-term expiratory airflow of infants born moderate-late preterm: a systematic review and meta-analysis. EClinicalMedicine 2022; 52: 101597.
43. Naumburg E, Söderström L. Increased risk of pulmonary hypertension following premature birth. BMC Pediatr 2019; 19: 288.
44. Gough A, Spence D, Linden M, Halliday HL, McGarvey LPA. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: a systematic review. Chest 2012; 141: 1554-1567.
45. Bui DS, Perret JL, Walters EH, et al. Association between very to moderate preterm births, lung function deficits, and COPD at age 53 years: analysis of a prospective cohort study. Lancet Respir Med 2022; 10: 478-484.
46. Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 2008; 32: 321-328.
47. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022; 400: 921-972.
48. Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD executive summary. Eur Respir J 2023; 61: 2300239.
49. Dani A, Hayes D Jr, Guzman-Gomez A, et al. Lung transplantation for bronchopulmonary dysplasia. Chest 2023; 163: 1166-1175.
50. Liu N, Cummings OW, Lagstein A, Hage CA, Chan KM, Zhang C. Lung transplantation for bronchopulmonary dysplasia in adults: a clinical and pathologic study of 3 cases. Am J Surg Pathol 2020; 44: 509-515.
51. Doyle LW, Irving L, Haikerwal A, Lee K, Ranganathan S, Cheong J. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax 2019; 74: 1147-1153.
52. Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990-2019. JAMA Pediatr 2022; 176: 787-796.
53. Lee SM, Sie L, Liu J, Profit J, Lee HC. Evaluation of trends in bronchopulmonary dysplasia and respiratory support practice for very low birth weight infants: a population-based cohort study. J Pediatr 2022; 243: 47-52.e2.
54. Perez A, Thiede L, Lüdecke D, Ebenebe CU, von dem Knesebeck O, Singer D. Lost in transition: health care experiences of adults born very preterm-a qualitative approach. Front Public Health 2020; 8: 605149.
55. Bolton CE, Bush A, Hurst JR, et al. Are early life factors considered when managing respiratory disease? A British Thoracic Society survey of current practice. Thorax 2012; 67: 1110.
56. Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA 2011; 306: 1233-1240.
57. Crump C. Medical history taking in adults should include questions about preterm birth. BMJ 2014; 349: g4860.
58. Kelly MM, Michalek R. Children born preterm: how are we educating providers? J Nurs Educ 2019; 58: 339-346.
59. Cassady SJ, Lasso-Pirot A, Deepak J. Phenotypes of bronchopulmonary dysplasia in adults. Chest 2020; 158: 2074-2081.
60. Duijts L, van Meel ER, Moschino L, et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J 2020; 55: 1900788.
61. Goulden N, Cousins M, Hart K, et al. Inhaled corticosteroids alone and in combination with long-acting β2 receptor agonists to treat reduced lung function in preterm-born children: a randomized clinical trial. JAMA Pediatr 2022; 176: 133-141.
62. Urs RC, Evans DJ, Bradshaw TK, et al. Inhaled corticosteroids to improve lung function in children (aged 6–12 years) who were born very preterm (PICSI): a randomised, double-blind, placebo-controlled trial. Lancet Child Adolesc Health 2023; 7: 567-576.