Respiratory syncytial virus infection: what’s available to prevent it and what’s coming?
Respiratory syncytial virus (RSV) is a leading cause of severe respiratory illness, particularly in infants, young children and older adults. Effective prevention strategies have been historically limited, but recent advancements, including the development of long-acting monoclonal antibodies, such as nirsevimab, and the approval of new vaccines for older adults and pregnant women, represent a paradigm shift in RSV infection prevention. By integrating these advances into existing healthcare frameworks and addressing ongoing challenges in vaccine development, we can significantly reduce the burden of RSV and improve outcomes for at-risk populations.
- Respiratory syncytial virus (RSV) impacts all age groups causing severe respiratory illness, with the greatest impact seen in infants, young children and older adults, leading to substantial morbidity and mortality.
- Nirsevimab, a newly developed long-acting monoclonal antibody, has demonstrated high efficacy in preventing RSV-related hospitalisations among infants.
- Recently approved vaccines, Arexvy and Abrysvo, have shown high efficacy in preventing RSV infections in older adults as well as pregnant women.
- Pioneering approaches, such as messenger RNA and nanoparticle-based vaccines, along with next-generation monoclonal antibodies, are being developed to provide more robust and long-lasting protection against RSV infection.
- Ensuring equitable access to RSV infection preventive measures is essential for maximising their impact. Public health initiatives must focus on uniform nationwide funding, policy recommendations and collaborations to address disparities, particularly among Aboriginal and Torres Strait Islander people.
- Continued research is necessary to address critical gaps in understanding the immunopathogenesis of RSV infection, viral evolution and long-term immunity. Developing novel antiviral therapies will be key to controlling RSV infection comprehensively.
Respiratory syncytial virus (RSV) is a significant global health concern, recognised as one of the leading causes of severe respiratory illness across all age groups. It disproportionately affects infants, young children and older adults.1,2 First identified in humans in 1956,3 RSV has since been a pervasive and challenging pathogen because of its highly contagious nature and the severe disease it can cause (defined as requiring hospitalisation), especially in vulnerable populations. RSV is the primary cause of acute lower respiratory tract (LRT) infections in children aged younger than 5 years,1,4 resulting in substantial healthcare utilisation and economic burden.5,6
In light of the significant morbidity and mortality associated with RSV infection, especially among high-risk groups, its prevention has become a critical focus in public health. Over the past few decades, substantial advancements have been made in understanding the epidemiology, pathophysiology and immune evasion mechanisms of RSV, leading to the development of innovative preventive strategies.
The objective of this article is to highlight the effectiveness of these novel preventive strategies and explore their potential to transform RSV infection management in the near future. By examining recent advances, such as the introduction of long-acting monoclonal antibodies, such as nirsevimab, and newly approved vaccines targeting older adults and pregnant women, this article outlines the pivotal shifts occurring in RSV infection prevention. Additionally, it will address the critical need for equitable access to these innovations and the role of public health initiatives in ensuring broad and effective implementation.
Virology and pathophysiological effects of RSV
Human RSV, classified as Orthopneumovirus hominis, is an enveloped virus with a negative- sense, single-stranded RNA genome about 15.2 kilobases in length.7 It belongs to the Pneumoviridae family, which also includes the human metapneumovirus.8 The RSV genome encodes 11 proteins, including three surface glycoproteins that are crucial for viral entry into the host cell: the fusion (F) protein, the attachment (G) protein and the small hydrophobic (SH) protein.9
The F protein is pivotal in facilitating the fusion of the viral envelope with the host cell membrane, a process that allows the viral genome to enter the cytoplasm and initiate infection. The G protein mediates the attachment of the virus to host cells, whereas the SH protein plays a role in viral assembly and budding.10 Understanding the structure and function of these proteins, particularly the F protein, has been central to the development of targeted therapies and vaccines against RSV. For instance, the stabilisation of the F protein in its prefusion conformation has been a key innovation in both vaccine development and the creation of monoclonal antibodies, such as nirsevimab, which are designed to neutralise the virus.
RSV is classified into two major subtypes, RSV-A and RSV-B, based on differences in the G protein.11,12 These subtypes circulate concurrently, but their prevalence can vary by season and geography. Both subtypes are associated with significant morbidity and mortality.13-16
Infection begins when RSV attaches to ciliated epithelial cells of the upper respiratory tract via the G protein.10 The F protein then facilitates fusion with the host cell membrane, enabling the viral RNA to enter and replicate within the host cell. This triggers a robust immune response, including the activation of innate immune pathways and production of proinflammatory cytokines.17 However, RSV has evolved several mechanisms to evade the host immune response. For example, the viral NS1 and NS2 proteins inhibit interferon signalling pathways, diminishing the effectiveness of the antiviral response.18 Additionally, the G protein acts as a decoy, binding to chemokine receptors and preventing the recruitment of immune cells to the site of infection.10
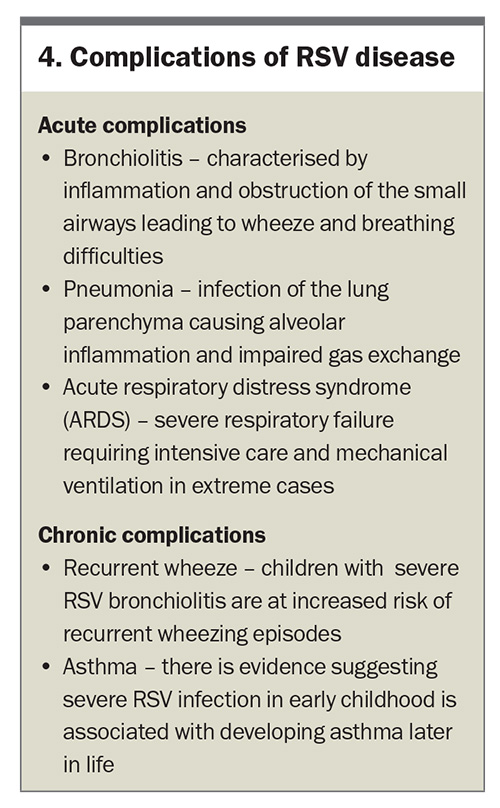
The initial infection in the upper respiratory tract can quickly spread to the LRT, leading to more severe conditions such as bronchiolitis and pneumonia. Bronchiolitis is characterised by inflammation and obstruction of the small airways, leading to wheezing, coughing and respiratory distress. Pneumonia, on the other hand, involves inflammation of the alveoli, which impairs gas exchange and can result in severe respiratory complications.19 The immune response is essential for clearing the virus, but can also exacerbate the disease.10,16 Excessive production of proinflammatory cytokines and chemokines can lead to an exaggerated inflammatory response, causing further damage to the respiratory epithelium.17
The long-term consequences of RSV infection, particularly in infants, can be significant. Severe RSV bronchiolitis in early childhood is associated with recurrent wheezing and the development of asthma later in life.20,21 It is hypothesised that persistent inflammation and remodelling of the airways following acute infection may play a role in the pathogenesis of this.22
Global and Australian incidence, prevalence and seasonality
RSV is a ubiquitous health concern, responsible for an estimated 33 million episodes of acute LRT infections annually in children aged younger than 5 years worldwide. These infections result in about 3.2 million hospitalisations and 118,200 deaths each year.23 However, these figures likely underestimate the true extent of RSV-related disease, as they do not account for older children24 and rely on hospital coding-based diagnosis.25 Additionally, there is substantial under-reporting from low- and middle-income countries.1,26
The highest burden of severe disease and mortality is observed in infants, especially those aged younger than 6 months, and older adults aged 65 years and older.1,2,27 Globally, almost all individuals will have experienced an RSV infection by the age of 2 years, but because infection does not confer lifelong immunity, reinfection is common across the lifespan.
RSV infection exhibits strong seasonal patterns. In temperate climates, RSV activity typically peaks during the winter months,28 whereas tropical and subtropical regions experience year-round RSV activity with one or two peaks depending on local climatic conditions.29 These variations highlight the importance of tailoring prevention strategies to the specific epidemiological context of each region.
In Australia, RSV has long been recognised as a significant cause of LRT infection-related hospitalisations, particularly among children. However, it was only in 2021 that RSV infection became a notifiable disease, with data collection integrated into the National Notifiable Disease Surveillance System (NNDSS). Prior to this, RSV-related data were largely derived from state-based studies and hospital records.
Recent studies have confirmed that RSV remains the most frequent cause of LRT infection-related hospitalisations among Australian children.30 A data linkage study undertaken in New South Wales (NSW) found an RSV-related hospitalisation incidence of 4.9 per 1000 child-years among those aged younger than 5 years, with a peak incidence of 25.6 per 1000 child-years among infants aged up to 3 months.31 Aboriginal and Torres Strait Islander children are disproportionately affected, with hospitalisation rates two to four times higher than in non-Indigenous children.25,31-33 This disparity underscores the urgent need for targeted public health interventions.
The prevalence of RSV infection in Australia typically peaks during the late autumn and winter months (May to August) in temperate regions. In contrast, tropical areas (such as north Queensland and parts of the Northern Territory) experience a more prolonged RSV infection season, with peaks from November to April.34-38 Understanding these seasonal variations is crucial for optimising the timing of prophylactic measures, such as vaccine administration and monoclonal antibody treatments.
Recent epidemiological trends
Recent trends indicate a rising awareness and diagnosis of RSV infections, partly because of improved diagnostic techniques and surveillance systems. The coronavirus disease (COVID-19) pandemic initially led to decreased RSV circulation, likely because of public health measures such as mask-wearing and social distancing.39-41 However, subsequent seasons have seen increased RSV activity as these measures were relaxed.42-44
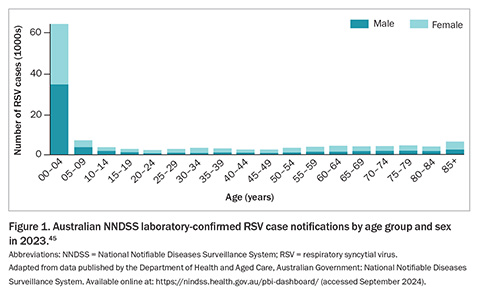
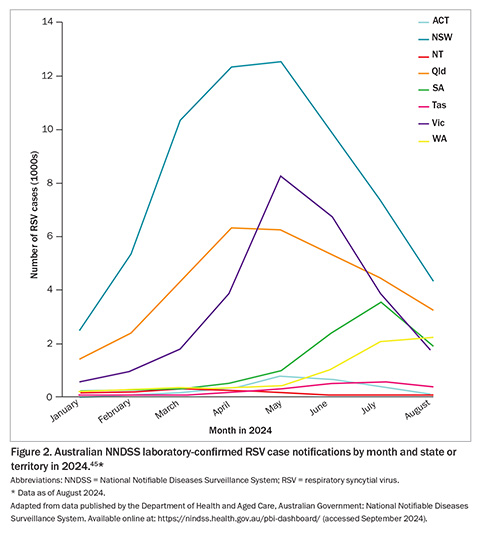
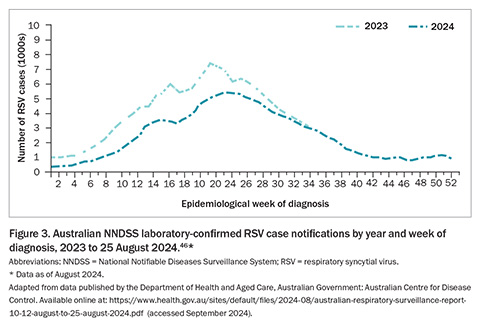
In 2023, the Australian NNDSS received 128,110 confirmed RSV infection case notifications, with 50.3% of cases occurring in children aged younger than 5 years, and 21.5% in individuals aged 60 years and older (Figure 1).45 By the end of August 2024, Australia had already recorded 149,042 RSV notifications, with infection cases appearing to decrease or plateau across all jurisdictions except Western Australia (WA) over the preceding month (Figure 2).45 Similar to the trends observed in 2023, RSV notifications followed an increasing trend from January this year through to an apparent peak in late May 2024 and have since followed a decline (Figure 3).46 These figures highlight the ongoing burden of RSV and the importance of continued surveillance and public health preparedness.
Populations at increased risk of severe RSV infection
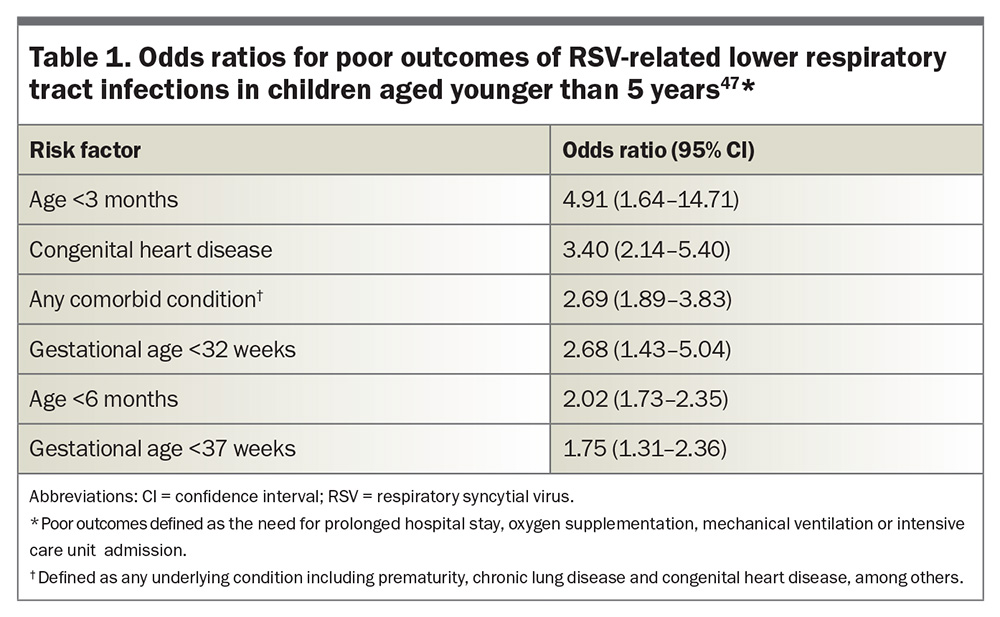
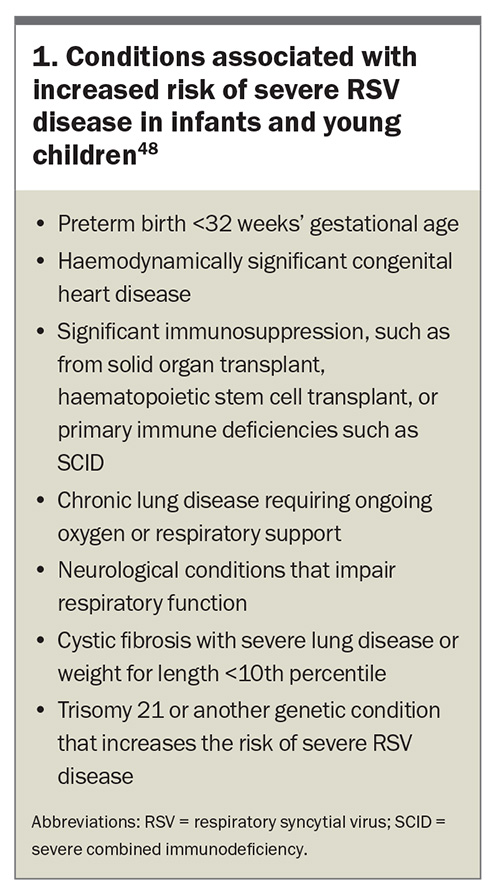
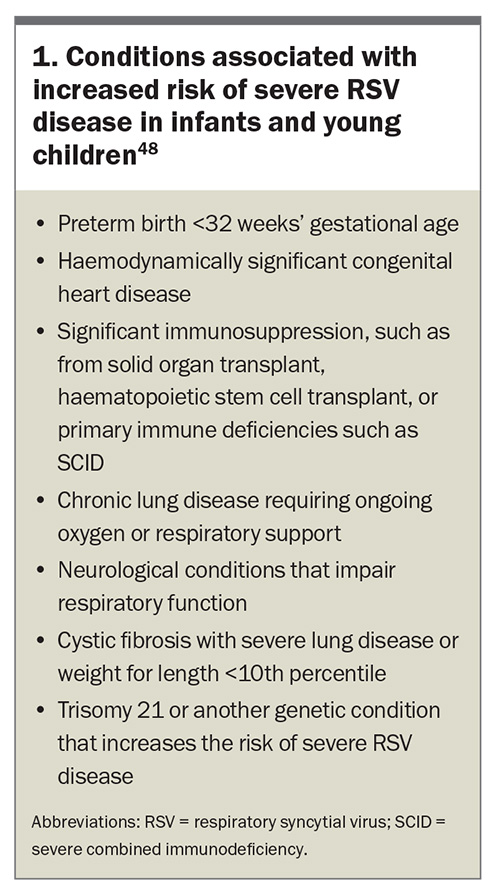
Infants, particularly those aged younger than 6 months, are at the highest risk of severe RSV disease.1,31 A 2022 systematic review and meta-analysis found that preterm infants (gestational age <37 weeks) and those with haemodynamically significant congenital heart disease, chronic lung disease of prematurity and immunocompromised states are particularly vulnerable to severe complications from RSV infection. The odds ratios for poor outcomes of RSV-related LRT infections in children aged younger than 5 years are presented in Table 1.47 The conditions associated with an increased risk of severe RSV disease in infants and young children, as outlined in the Australian Immunisation Handbook, are listed in Box 1.48 The incidence of RSV hospitalisation was also found to be higher between late autumn and late winter, with a peak between early to mid-winter, reaching a hospitalisation rate of 11.6 cases per 1000, and a decreased incidence rate throughout the summer (about 0.5 cases per 1000).
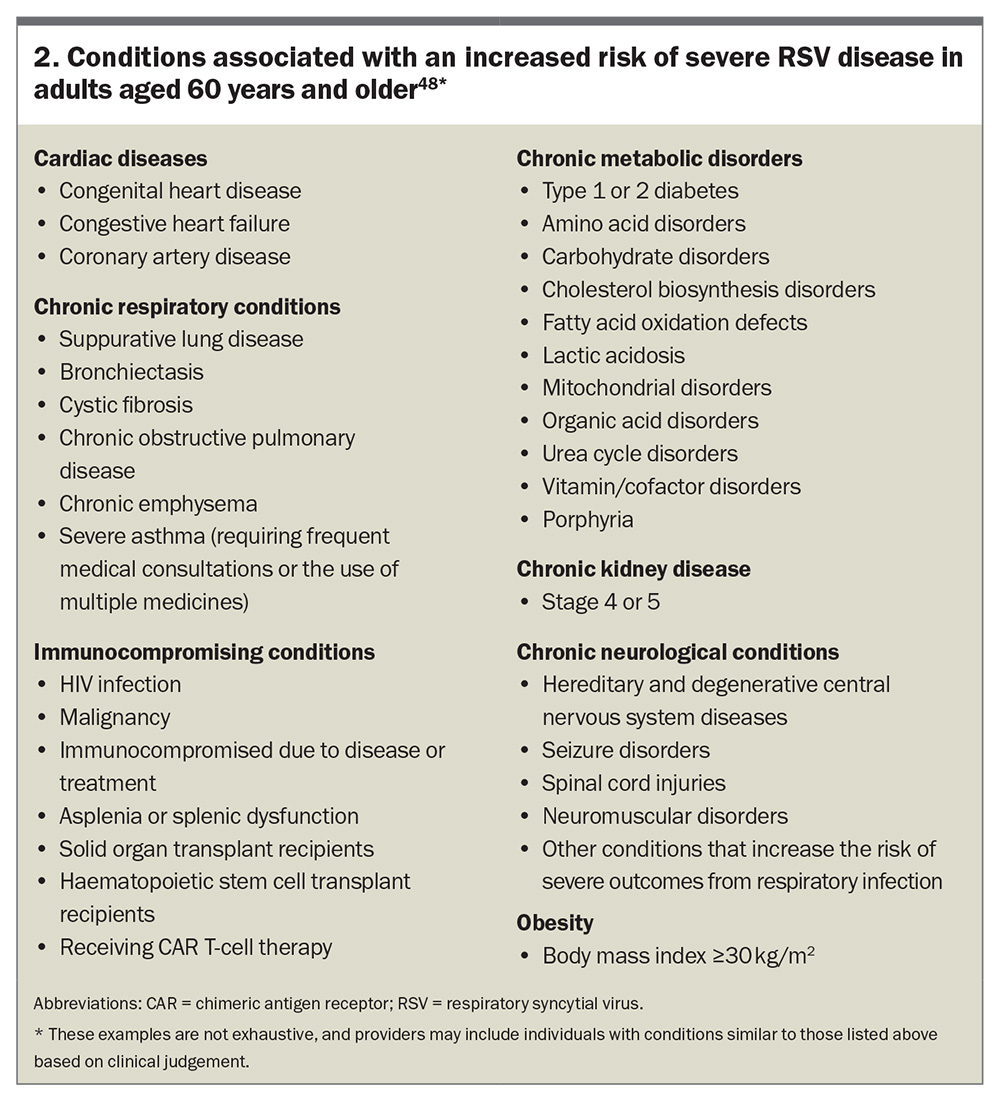
The decline in immune function with age (immunosenescence) likely contributes to the increased susceptibility and severity of RSV infections in older adults. Conditions associated with an increased risk of severe RSV disease in adults aged 60 years and older, as outlined in the Australian Immunisation Handbook, are listed in Box 2.48 These patients are more likely to require intensive care and have prolonged illness durations.
Symptomatology and complications of RSV infection
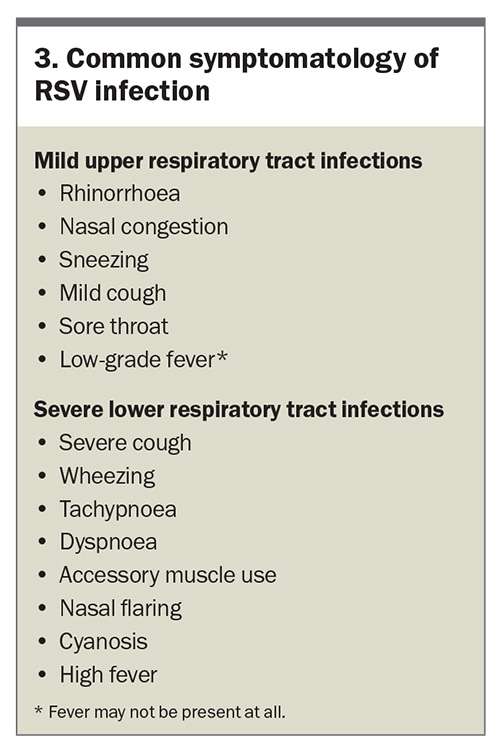
RSV infection can present with a wide range of clinical manifestations, varying from mild upper respiratory tract infections to severe LRT infections (Box 3). The severity of symptoms often depends on the patient’s age, immune status and underlying health conditions. RSV infection typically begins with symptoms affecting the upper respiratory tract, resembling those of a common cold and that are often self-limiting in healthy adults and older children. In infants and older adults, RSV can progress to the LRT and cause conditions such as bronchiolitis and pneumonia, sometimes with lasting consequences (Box 4).
Prevention starts with fundamental hygiene practices
Basic hygiene and infection control measures play a fundamental role in preventing the spread of acute respiratory infections, including RSV infection.49 These include:
- frequent hand hygiene (use of alcohol-based hand sanitiser is the standard of care for hand hygiene in Australian healthcare settings, whereas handwashing is reserved for situations when hands are visibly soiled, or when gloves have not been worn in the care of patients with Clostridioides difficile infection)50
- regular cleaning of surfaces and objects that may be contaminated with the virus
- minimising close contact with suspected or known infected individuals
- covering the mouth and nose with a tissue or elbow when coughing or sneezing to prevent the spread of respiratory droplets containing the virus.
- Passive immunisation for infants and young children – monoclonal antibodies
- Passive immunisation using monoclonal antibodies provides immediate but temporary protection against RSV.
Palivizumab
Palivizumab is a humanised immunoglobulin G1 monoclonal antibody that has been used since 1998 to prevent severe RSV disease in high-risk infants, including those with bronchopulmonary dysplasia, haemodynamically significant congenital heart disease or born prematurely (gestational age ≤35 weeks at birth).51 Administered as monthly intramuscular injections during the RSV season, palivizumab has been shown to reduce hospitalisations associated with RSV by about 58% (95% confidence interval [CI], 43.1–69%).52,53 Unfortunately, given the drug’s high cost and suboptimal dosing regimen, it is not a feasible strategy to prevent RSV disease at the population level.54
Nirsevimab
The approach to RSV prevention has been recently transformed with the introduction of nirsevimab, an injectable recombinant human immunoglobulin G1 kappa monoclonal antibody engineered with a mutation in the Fc domain to extend its serum half-life.55,56 A single dose of this drug allows for effective prophylaxis for at least five months.57 Nirsevimab targets the prefusion F glycoprotein of RSV, a key element necessary for viral entry into host cells, neutralising the virus and preventing disease.58,59 This breakthrough is based on a detailed understanding of the prefusion F glycoprotein’s structure and function. The stabilisation of the prefusion F glycoprotein has been pivotal, inducing a stronger immune response and providing enhanced protection against RSV infection.
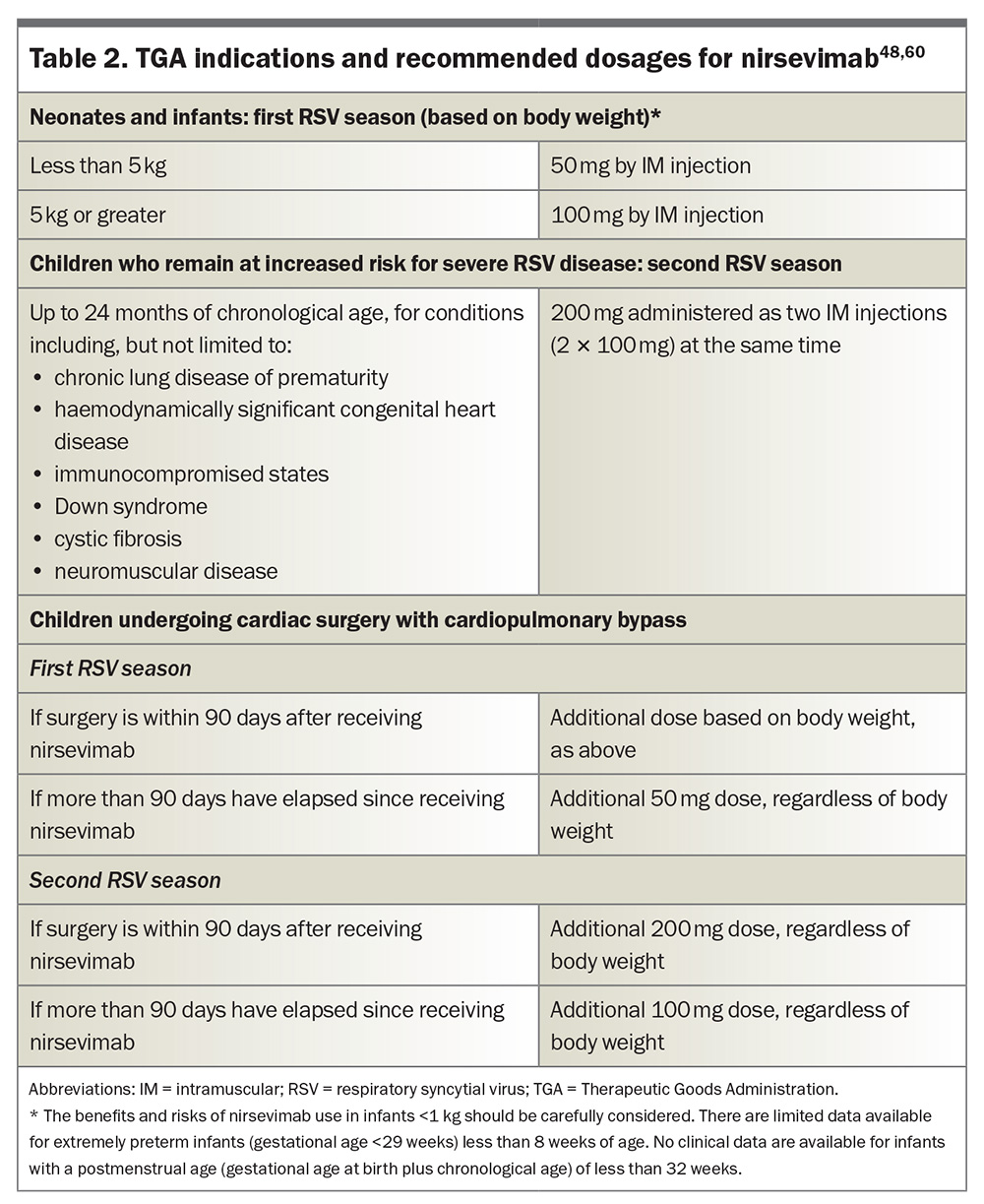
Nirsevimab was TGA approved in November 2023 for the prevention of RSV LRT disease in:60
- neonates and infants born during or entering their first RSV season
- children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season (see Box 1).
Efficacy and safety
The efficacy and safety of nirsevimab in preventing medically attended (MA) RSV LRT infection in both term and preterm infants during their first RSV season was evaluated in two randomised, double-blind, placebo-controlled, multicentre trials – D5290C00003 (phase 2b) and MELODY (phase 3) – which included sites in Australia.57,61 In the phase 2b trial, nirsevimab prophylaxis resulted in a 70.1% reduction in the incidence of MA RSV LRT infection compared with placebo, and a 78.4% reduction in RSV-related hospitalisations (0.8% vs 4.1% of infants).61 The MELODY trial further showed a 74.5% reduction in MA RSV LRT infections and a 62.1% reduction in hospitalisations in the nirsevimab group (0.6% vs 1.6% in healthy late preterm and term infants) compared with placebo.57 The safety profile of nirsevimab was shown to be favourable, with comparable rates of adverse events across treatment groups in the phase 2–3 MEDLEY trial, which compared nirsevimab with palivizumab in high-risk infants.62 Common adverse reactions included rash (0.7%), pyrexia (0.5%) and mild injection-site reactions (0.3%) shortly after dosing.
Further evidence of nirsevimab’s efficacy was provided by the HARMONIE study, a phase 3b real-world trial involving 8058 infants, which demonstrated an 83.2% reduction in RSV-related hospitalisations and a 75.7% reduction in very severe RSV LRT infections.63 Early implementation programs in several European countries and the USA have corroborated the efficacy observed in clinical trials.64-70 A recent population-based study in Galicia, Spain, also supported these findings, revealing an 82.0% effectiveness against RSV-related LRT infection hospitalisations and an 86.9% effectiveness against severe RSV LRT infections requiring oxygen support under real-world conditions.71 Additionally, follow up of the MELODY trial alleviated concerns about enhanced disease during the second RSV season following nirsevimab administration, showing a continued low incidence of RSV infection and no increase in disease severity compared with placebo.72
Dosing and administration
Nirsevimab is available as a 50mg solution in a 0.5mL prefilled syringe (purple plunger rod), and as a 100mg solution in a 1mL prefilled syringe (light blue plunger rod); needles are not included.60 The TGA indications and recommended dosages for nirsevimab are listed in Table 2.48,60 Nirsevimab can be co-administered with other childhood vaccines, although specific studies on drug–drug interactions mediated by nirsevimab have not been conducted, and there are no available data on its co-administration with other immunoglobulin products.54,60 Importantly, the administration of palivizumab is contraindicated in infants who have already received nirsevimab during the same RSV season. However, nirsevimab can be administered before or during a second RSV season in children up to 24 months of age who are still at risk of severe RSV disease and who received palivizumab during their first RSV season.60 Moreover, the US Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics recommend that infants who initially received fewer than five doses of palivizumab in a season should receive a single dose of nirsevimab instead of additional doses of palivizumab.73,74 There is no required minimum interval between the last dose of palivizumab and the administration of nirsevimab. However, because the protective effect of palivizumab diminishes after 30 days, it is recommended to administer nirsevimab within 30 days following the final dose of palivizumab, whenever possible, to ensure continuous protection against RSV. Refer to the Australian Product Information for full details.60
As of September 2024, nirsevimab has been made available in only three Australian states. WA and Queensland have implemented free, large-scale programs open to all newborns, as well as infants at increased risk of severe RSV disease due to complex medical conditions. In contrast, NSW offers nirsevimab only to certain vulnerable infants. In WA and Queensland, nirsevimab is also available from GPs and routine immunisation providers. However, in NSW, nirsevimab is only available for eligible infants through treating hospitals. Detailed information is available from the WA, Queensland and NSW program websites.75-77
Despite its availability in these states, at the July 2024 meeting of the Pharmaceutical Benefits Advisory Committee (PBAC), nirsevimab was not recommended for General Schedule Restricted Benefit PBS listing.78 Although the PBAC acknowledged that nirsevimab is superior to the absence of immunisation in terms of effectiveness, with an acceptable safety profile in the first RSV season, they raised concerns about the incremental cost-effectiveness ratio, deeming it to be substantially underestimated and highly uncertain. Additionally, the PBAC did not accept palivizumab as the main comparator for use in the second RSV season, citing limited clinical evidence to support nirsevimab’s proposed listing for this indication.
Active immunisation for pregnant women and older adults – vaccines
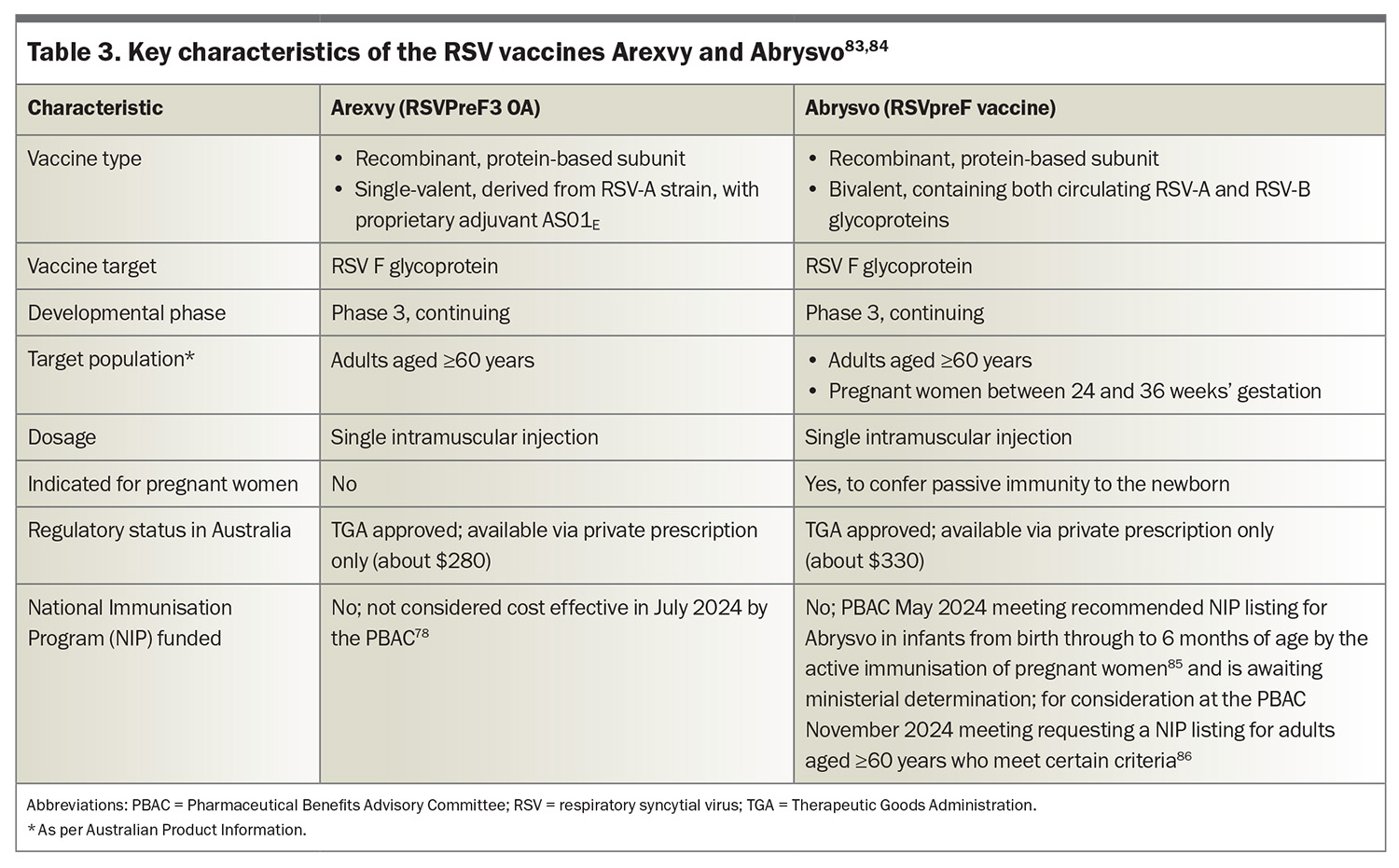
Arexvy (RSVPreF3 OA) and Abrysvo (RSVpreF vaccine) received TGA approval in January and April 2024, respectively, heralding a new era in RSV infection prevention.79,80 Both Arexvy and Abrysvo target the prefusion F glycoprotein of RSV, with Arexvy incorporating an adjuvant (AS01E) to boost the immune response in older patients who may experience immunosenescence; the key features of both vaccines are presented in Table 3.78,81-86 These vaccines are designed to elicit strong immune responses that protect against RSV infection in older adults, and in the case of Abrysvo, also provide immunity to newborns through placental transfer when administered to pregnant women.81
In Australia, Arexvy and Abrysvo are indicated for the prevention of LRT disease associated with RSV infection in adults aged 60 years and older,83 and Abrysvo is also approved for the same older age group and pregnant women between 24 and 36 weeks’ gestation to protect newborns from birth through 6 months of age.84 The new chapter in the Australian Immunisation Handbook recommends RSV vaccination for:
- all adults aged 75 years and older
- Aboriginal and Torres Strait Islander people aged 60 years and older
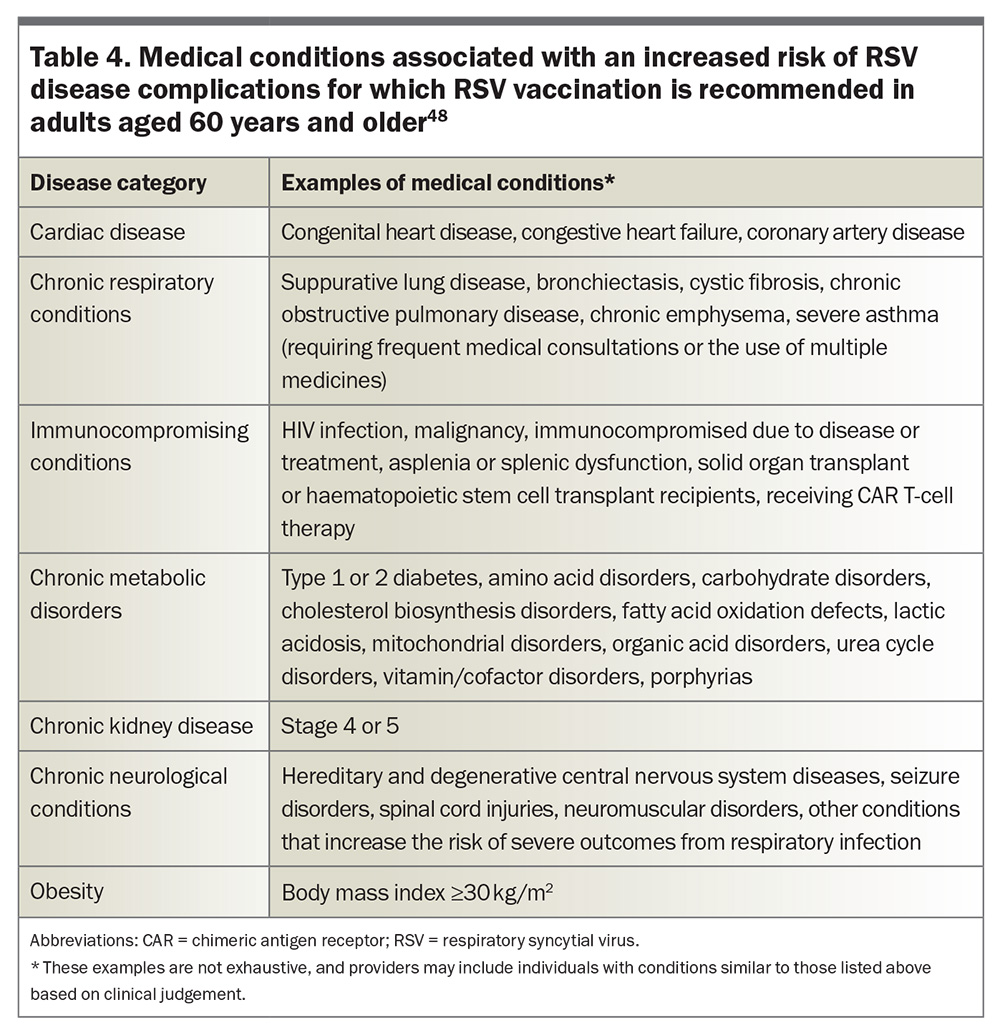
- adults aged 60 years and older with risk factors for severe RSV disease (Table 4)
- pregnant women at 28 to 36 weeks’ gestation to protect their newborn infants.48
Other adults aged 60 to 74 years may consider vaccination, bearing in mind the benefits may be reduced.
Efficacy and safety
In a continuing phase 3 clinical trial, a single dose of Arexvy in adults aged 60 years and older (n=24,966 at interim analysis) showed 82.6% efficacy in preventing symptomatic, laboratory-confirmed RSV LRT disease, 94.1% efficacy against severe RSV LRT disease and 71.7% efficacy against RSV-associated acute respiratory infection, compared with placebo.87 The vaccine’s protective effects lasted throughout the first RSV season (median follow up: 6.7 months) and the vaccine was well tolerated, with mild to moderate injection-site reactions and systemic symptoms, such as fatigue and headache, being the most common side effects. Recent findings from the second RSV season (up to 22 months postvaccination) indicated a modest decline in efficacy: 67.2% against laboratory-confirmed RSV LRT disease and 78.8% against severe RSV LRT disease.88 Revaccination at the start of the second season did not affect efficacy.
Similarly, Abrysvo demonstrated strong efficacy and safety in adults aged 60 years and older in the ongoing phase 3 RENOIR trial.89 After the first RSV season (n=34,284; mean follow up, seven months), an interim analysis revealed 66.7% efficacy in preventing laboratory-confirmed RSV LRT infection with two or more signs or symptoms, 85.7% efficacy against RSV LRT infection with three or more signs or symptoms and 62.1% efficacy against RSV-associated acute respiratory disease, compared with placebo. Abrysvo was also well tolerated, with common side effects including mild to moderate injection-site reactions and fatigue. Unpublished data from a recent Pfizer press release revealed 65.1% efficacy against two or more signs or symptoms after the first season, and 55.7% after the second season.90 The efficacy against three or more signs or symptoms was 88.9% after the first season and 77.8% after the second.
An estimated reduction in severe disease and hospitalisations following Arexvy and Abrysvo vaccination would likely be significant, according to data presented at the June 2024 meeting of the US ACIP.91 Modelling over two consecutive RSV seasons showed Arexvy preventing about 4283 hospitalisations, 630 intensive care unit admissions and 605 deaths per one million vaccine doses administered to adults aged 75 years and older. Likewise, Abrysvo would avert around 3817 hospitalisations, 561 intensive care unit stays and 539 deaths per one million doses administered in the same age group. Similar, although less impressive, results were also seen in adults aged 65 to 74 years who were at increased risk of severe RSV disease.
However, there have been concerns about the potential association between RSV vaccines and Guillain–Barré syndrome (GBS). Clinical trials identified GBS as a potential safety concern, and postmarketing surveillance has revealed GBS cases at rates of 4.4 and 1.8 per one million doses of Abrysvo and Arexvy, respectively, which are higher than expected background rates.92 The Vaccine Adverse Event Reporting System, V-safe, the Vaccine Safety Datalink Project and the Clinical Immunization Safety Assessment Network are surveillance programs of the US Centers for Disease Control and Prevention; together with the Food and Drug Administration (FDA), they are used to monitor for unusual or unexpected patterns of vaccine-related adverse event reports that may indicate a possible safety concern. In considering the available data, the FDA maintains that the benefits of Arexvy and Abrysvo vaccination in preventing RSV-related hospitalisation outweigh the potential risks associated with the vaccines.93
Additionally, the phase 3 GRACE (RSV MAT-009) trial conducted in pregnant women raised concerns regarding the potential link between Arexvy and an increased risk of preterm birth (defined as birth at less than 37 weeks’ gestation).94 When the independent data monitoring committee reported the safety concern, enrolment was immediately halted. A subsequent analysis showed an overall increased incidence of preterm births in the vaccine group (6.8% vs 4.9% in the placebo group; relative risk, 1.37; 95% CI, 1.08–1.74; p = 0.01), and the trial was discontinued. No definitive mechanism has been identified despite extensive post-hoc analyses.
Conversely, prenatal vaccination with Abrysvo was not associated with a statistically significant increased risk of preterm birth (5.7% vs 4.7% in the placebo group),95 nor in a retrospective observational cohort study (5.9% vs 6.7% in the placebo group), even when adjusted for potential confounders and addressing immortal time bias.96 Furthermore, although no significant differences in pregnancy and neonatal outcomes based on vaccination status were seen, an increased risk of hypertensive disorders of pregnancy was observed (hazard ratio, 1.43; 95% CI, 1.16–1.77) in the observational study only.
Dosing and administration
The Arexvy single-dose vaccine contains a lyophilised RSV glycoprotein prefusion F stabilised in the prefusion conformation, along with a liquid suspension containing a proprietary AS01E liposome-based adjuvant. After reconstitution, each 0.5mL dose delivers 120 micrograms of RSVPreF3 antigen adjuvanted with AS01E. The Abrysvo single-dose vaccine includes a sterile water diluent, a lyophilised RSVpreF vaccine, and a vial adapter. After reconstitution, each 0.5mL dose provides 60micrograms of stabilised prefusion F glycoproteins from both RSV subgroups A and B.
In older adults, both Arexvy and Abrysvo can be co-administered with other vaccines, including vaccines for severe acute respiratory syndrome coronavirus 2, seasonal influenza, pneumococcal and recombinant zoster (Shingrix).48 Recent safety and immunogenicity data on co-administered seasonal adjuvanted quadrivalent influenza vaccine with Abrysvo and Arexvy showed noninferiority of immune responses with acceptable safety and tolerability profiles.97,98 However, increased local (53% vs 40%) and systemic adverse events (45% vs 34%) were reported when compared with RSV vaccines alone.98 Clinicians should, therefore, weigh up the benefits of administering both vaccines at the same time against these concerns.48
What is best for newborns – maternal immunisation or nirsevimab?
When it comes to protecting newborns against RSV infection, both maternal immunisation and nirsevimab present effective strategies, each with distinct advantages.
In pregnant women, Abrysvo induces the production of antibodies that are transferred to the fetus through the placenta, offering passive immunity to the newborn that persists through the first few months of life.95 This approach may be particularly beneficial when integrated into existing antenatal programs. The timing of vaccination during pregnancy is crucial to ensure optimal antibody transfer.99 The Australian Immunisation Handbook recommends Abrysvo be given between 28 and 36 weeks’ gestation, as a precaution while awaiting further data on adverse events with administration from 24 to less than 28 weeks’ gestation.48 Maternal immunisation also provides dual protection, safeguarding both the mother and the infant against RSV infection.
In newborns, nirsevimab provides immediate and sustained protection against RSV infection for at least five months with a single dose.57 Unlike maternal immunisation, nirsevimab’s efficacy is independent of the timing of pregnancy or the mother’s immune response, making it a reliable option, especially for infants at high risk of severe RSV disease.100 Nirsevimab is particularly suited for infants born during the RSV season or for those with underlying health conditions that increase their susceptibility to severe RSV infection outcomes (Box 1).
The choice between maternal immunisation and nirsevimab depends on various factors, including the timing of pregnancy, immunisation product availability and the newborn’s risk profile. Maternal immunisation offers broad protection and ease of integration into existing health protocols, and nirsevimab provides targeted and reliable protection for high-risk infants. Combining both strategies, where appropriate, could offer the most comprehensive protection against RSV during the critical early months of life, minimising RSV-related morbidity and mortality among infants and young children.
Emerging technology and future directions
The landscape of RSV infection prevention is rapidly evolving, driven by significant advancements in vaccine technology, monoclonal antibody development and antiviral prophylaxis. These innovations offer promising new tools for combatting RSV infection.
Messenger RNA vaccine technology
The success of messenger RNA (mRNA) vaccines against COVID-19 has paved the way for similar innovations in RSV infection prevention. mRNA vaccines are designed to instruct cells to produce viral proteins that trigger an immune response, without using a live virus. This platform offers several advantages, including rapid development and the ability to update the vaccine to address new viral variants. In late May 2024, Moderna’s mRNA-based RSV vaccine, mRESVIA, was approved by the US FDA for adults aged 60 years and older, marking a significant milestone.101 Early trials have shown promising efficacy,102-104 although longer-term studies are needed to assess its durability and effectiveness across different age groups. Following recent positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use recommending marketing authorisation for mRESVIA in the European Union (pending European Commission approval), Moderna is preparing additional approval applications in multiple countries, including Australia. For clinicians, the arrival of mRNA-based RSV vaccines represents a new frontier in immunisation, with the potential to significantly reduce RSV-related hospitalisations among older adults.
Nanoparticle-based vaccines
Nanoparticle-based vaccines use virus-like particles to mimic the structure of RSV, thereby eliciting a strong and specific immune response.105 The enhanced stability and targeted delivery offered by nanoparticles could lead to more durable protection against RSV infection. Current trials are exploring their effectiveness in both paediatric and older populations. For clinicians, the future availability of nanoparticle vaccines could provide an additional option, particularly for patients who may not respond as well to traditional vaccines.
Next-generation monoclonal antibodies
Next-generation monoclonal antibodies are being developed to offer even longer-lasting immunity and broader protection against diverse RSV strains than nirsevimab.106 These advancements could further reduce the burden on healthcare systems during peak RSV seasons, allowing GPs to focus on preventive care for at-risk populations.
Antiviral prophylaxis
New oral antiviral drugs, such as sisunatovir (RV521)and rilematovir (JNJ-53718678),have shown efficacy in early trials by inhibiting the fusion of RSV with host cells, thereby reducing the viral load and symptom severity.107-109 These agents may soon provide an additional layer of protection, particularly for patients who are not candidates for vaccines or monoclonal antibodies. For clinicians, the introduction of antivirals could offer flexibility in managing RSV infection in high-risk or immunocompromised patients.
Future directions and research priorities
The focus of RSV research will likely shift towards optimising the delivery and durability of preventive measures. This includes enhancing the immunogenicity of vaccines, extending the duration of protection provided by monoclonal antibodies and developing new antiviral agents. Additionally, research into biomarkers and rapid diagnostics will be essential for early detection and targeted treatment of RSV infection.
Public health and policy implications
The full impact of the innovations discussed in this article will only be realised through effective public health policies, equitable access and comprehensive implementation strategies. For Australia, this means addressing unique local challenges while ensuring that these new tools are accessible to all populations, particularly those people most at risk.
Ensuring equitable access to RSV infection preventive measures is a critical public health priority. Australia’s diverse population includes groups who are disproportionately affected by RSV, such as Aboriginal and Torres Strait Islander people, people in rural and remote communities and those with socioeconomic disadvantages. These populations often face barriers to healthcare access, including a limited availability of medical services, geographic isolation and financial constraints. To address these disparities, public health policies must focus on the following strategies.
Targeted outreach and education
Public health campaigns should be tailored to reach high-risk communities, emphasising the importance of RSV infection prevention and the availability of new vaccines and monoclonal antibodies. Collaboration with local healthcare providers, community leaders and Indigenous health organisations will be key to ensuring that these messages resonate and lead to increased uptake.
Funding and subsidisation
Equitable access requires that preventive measures be affordable and widely available. It is essential to advocate for the inclusion of these preventive measures in national funding schemes to ensure that high-risk populations are protected.
The PBS and the National Immunisation Program (NIP) are key funding mechanisms that could facilitate access to RSV vaccines, such as Arexvy and Abrysvo, and monoclonal antibodies like nirsevimab. However, the inclusion of these interventions is contingent upon rigorous evaluation by the PBAC, which assesses their clinical efficacy, safety and cost effectiveness. In May 2024, the PBAC recommended NIP listing for Abrysvo in infants from birth through to 6 months of age through the active immunisation of pregnant women between 28 and 36 weeks’ gestation.85 As of September 2024, a ministerial determination for its inclusion is pending. Abrysvo’s listing for adults aged 60 years and older who meet specific criteria will be reconsidered at the November 2024 meeting,86 after previously being denied NIP listing because of a high and uncertain incremental cost-effectiveness ratio, with the PBAC stating a price reduction would be required to ensure the vaccine was cost-effective in the proposed group.110
Including these preventive measures on the PBS and NIP would alleviate financial barriers, supporting physicians in recommending and administering these interventions to at-risk patients without concerns about cost implications. As seen in WA and Queensland, state-level funding initiatives can serve as potential successful models, demonstrating the feasibility and impact of broad-access programs. Advocating for government subsidies is about improving individual patient outcomes and reducing the overall healthcare burden associated with RSV, including excess emergency department visits, hospital admissions and intensive care unit stays. The potential savings in healthcare costs and improved patient quality of life make a compelling case for prioritising the inclusion of RSV vaccines and monoclonal antibodies in national funding schemes.
Implementation of vaccination programs
The successful implementation of RSV vaccination programs will depend on integrating these new vaccines into Australia’s existing immunisation infrastructure, ensuring broad coverage, particularly for high-risk groups. The Australian Immunisation Handbook has already begun incorporating recommendations for RSV vaccines, and ongoing updates will be necessary as new data emerge. Key considerations for implementation include the following.
Seasonal timing
Given the distinct RSV seasonality in different parts of Australia, timing the administration of vaccines and monoclonal antibodies will be crucial. In temperate regions, where RSV infections peak in the winter, vaccination efforts should be concentrated in the months leading up to the peak season. In tropical and subtropical regions, where RSV activity is more variable, local data should guide the timing of interventions.
Integration with existing programs
Leveraging existing immunisation programs, such as those for influenza and COVID-19, as well as standard pregnancy regimens, can streamline the delivery of RSV vaccines. Co-administration with other vaccines may also be possible, although clinicians should weigh the benefits against any potential increase in adverse events or decrease in vaccine efficacy, as seen with some co-administered vaccines.111
Policy recommendations for monoclonal antibody distribution
The distribution of monoclonal antibodies must be carefully managed to ensure that they reach those who need them most. Current programs in WA and Queensland have made nirsevimab widely accessible, but there are disparities between states regarding availability and eligibility criteria. To address these inconsistencies, the following policy recommendations should be considered.
National standardisation
A uniform national policy for the provision of nirsevimab is essential. This would ensure that all eligible infants across Australia, regardless of their location, have equitable access. Such a policy should be informed by the latest clinical data and guided by input from GPs, public health physicians, paediatricians and infectious diseases experts.
Expansion of access
Beyond targeting high-risk infants, as is the case in NSW, consideration should be given to expanding access to include broader groups, such as all infants born in RSV seasons as a minimum. This approach could help reduce the overall burden of RSV infection on healthcare systems.
Role of public health initiatives
Public health initiatives will be central to the success of RSV infection prevention strategies. Key components of these initiatives include the following.
Education and awareness campaigns
Public health campaigns should focus on educating parents, caregivers and healthcare providers about the risks of RSV infection and the benefits of prevention. These campaigns should also address common misconceptions and vaccine hesitancy, which could otherwise undermine efforts to achieve high coverage rates.
Community engagement
Engaging communities, particularly in remote and Indigenous areas, is crucial for rolling out RSV prevention measures successfully. Community health workers can play a vital role in promoting vaccination, facilitating access to preventive care and ensuring that local concerns and cultural practices are respected.
Monitoring and surveillance
Continuous surveillance of RSV activity is necessary to guide public health responses and assess the impact of preventive measures. The integration of RSV data into the NNDSS since 2021 is a positive step, but further enhancements in data collection, including real-time reporting and analysis, are needed. The establishment of the Australian Centre for Disease Control (ACDC) presents a significant opportunity to strengthen RSV monitoring and surveillance across the country. With its mandate to consolidate and streamline health data, the ACDC can play a crucial role in advancing RSV surveillance by implementing more robust real-time data collection systems, developing predictive modelling for RSV outbreaks, and enhancing the capacity to track vaccine efficacy and safety.112 By leveraging advanced analytics and integrating RSV data from hospitals, primary care and laboratory networks, the ACDC can provide clinicians and public health authorities with timely and actionable insights, ultimately supporting targeted interventions and optimising the management of RSV across Australia.
Conclusion
The prevention of RSV infection is entering a new era, marked by the introduction of innovative vaccines and monoclonal antibodies that offer effective protection across different age groups. These advancements hold the potential to transform the management of RSV, reducing the substantial morbidity and mortality associated with this virus. However, to fully realise the benefits of these innovations, it is crucial to address challenges related to equitable access, implementation and ongoing research. In Australia, targeted public health initiatives, standardised national policies and community engagement will be key to ensuring that these preventive measures reach all populations, particularly those most vulnerable to severe RSV disease. By embracing these emerging technologies and continuing to adapt our strategies, we can achieve comprehensive control of RSV and improve public health outcomes on both a national and global scale. RMT
COMPETING INTERESTS: Professor Griffin has received speaker honoraria from Seqirus. Dr Armstrong and Dr Spence: None.
References
1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390: 946-958.
2. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222 Suppl 7: S577-S583.
3. Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg 1957; 66: 281-290.
4. Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis 2018; 217: 1356-1364.
5. Li Y, Johnson EK, Shi T, et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9: 175-185.
6. Zhang S, Akmar LZ, Bailey F, et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis 2020; 222 Suppl 7: S680-S687.
7. Langedijk AC, Bont LJ. Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol 2023; 21: 734-749.
8. International Committee on Taxonomy of Viruses (ICTV). Current ICTV Taxonomy Release – Taxonomy Browser (2023 Release, MSL #39). ICTV; 2024. Available online at: https://ictv.global/taxonomy (accessed September 2024).
9. Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol 2013; 372: 3-38.
10. Battles MB, McLellan JS. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol 2019; 17: 233-245.
11. Anderson LJ, Hierholzer JC, Tsou C, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis 1985; 151: 626-633.
12. Mufson MA, Orvell C, Rafnar B, Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol 1985; 66: 2111-2124.
13. Nuttens C, Moyersoen J, Curcio D, et al. Differences between RSV A and RSV B subgroups and implications for pharmaceutical preventive measures. Infect Dis Ther 2024; 13: 1725-1742.
14. Saravanos GL, Ramos I, Britton PN, Wood NJ. Respiratory syncytial virus subtype circulation and associated disease severity at an Australian paediatric referral hospital, 2014-2018. J Paediatr Child Health 2021; 57: 1190-1195.
15. Vandini S, Biagi C, Lanari M. Respiratory syncytial virus: the influence of serotype and genotype variability on clinical course of infection. Int J Mol Sci 2017; 18: 1717.
16. Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus – a comprehensive review. Clin Rev Allergy Immunol 2013; 45: 331-379.
17. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30: 481-502.
18. Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol 2005; 79: 5353-5362.
19. Walsh EE, Englund JA. Respiratory syncytial virus. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020. p. 2093-2103.e6.
20. Binns E, Tuckerman J, Licciardi PV, Wurzel D. Respiratory syncytial virus, recurrent wheeze and asthma: a narrative review of pathophysiology, prevention and future directions. J Paediatr Child Health 2022; 58: 1741-1746.
21. Shi T, Ooi Y, Zaw EM, et al. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis 2020; 222 Suppl 7: S628-S633.
22. Su P, Jiang C, Zhang Y. The implication of infection with respiratory syncytial virus in pediatric recurrent wheezing and asthma: knowledge expanded post-COVID-19 era. Eur J Clin Microbiol Infect Dis 2024; 43: 403-416.
23. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399: 2047-2064.
24. Goldstein E, Nguyen HH, Liu P, et al. On the relative role of different age groups during epidemics associated with respiratory syncytial virus. J Infect Dis 2018; 217: 238-244.
25. Saravanos GL, Sheel M, Homaira N, et al. Respiratory syncytial virus-associated hospitalisations in Australia, 2006-2015. Med J Aust 2019; 210: 447-453.
26. Zanone SM, Krause LK, Madhi SA, et al. Challenges in estimating RSV-associated mortality rates. Lancet Respir Med 2016; 4: 345-347.
27. Wang X, Li Y, Shi T, et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data. Lancet 2024; 403: 1241-1253.
28. Price RHM, Graham C, Ramalingam S. Association between viral seasonality and meteorological factors. Sci Rep 2019; 9: 929.
29. Shan S, Zhang W, Gao H, et al. Global seasonal activities of respiratory syncytial virus before the coronavirus disease 2019 pandemic: a systematic review. Open Forum Infect Dis 2024; 11: ofae238.
30. Lim FJ, Blyth CC, Fathima P, de Klerk N, Moore HC. Record linkage study of the pathogen-specific burden of respiratory viruses in children. Influenza Other Respir Viruses 2017; 11: 502-510.
31. Homaira N, Oei JL, Mallitt KA, et al. High burden of RSV hospitalization in very young children: a data linkage study. Epidemiol Infect 2016; 144: 1612-1621.
32. Fathima P, Blyth CC, Lehmann D, et al. The impact of pneumococcal vaccination on bacterial and viral pneumonia in Western Australian children: record linkage cohort study of 469589 births, 1996-2012. Clin Infect Dis 2018; 66: 1075-1085.
33. Fagan P, McLeod C, Baird RW. Seasonal variability of respiratory syncytial virus infection in the Top End of the Northern Territory (2012-2014). J Paediatr Child Health 2017; 53: 43-46.
34. Paynter S, Ware RS, Sly PD, Weinstein P, Williams G. Respiratory syncytial virus seasonality in tropical Australia. Aust N Z J Public Health 2015; 39: 8-10.
35. Nixon JC, Freeman K, Baird RW. Altered epidemiological patterns of respiratory syncytial virus and influenza detections in a tropical Australian setting 2020 to 2023. Aust N Z J Public Health 2024; 48: 100172.
36. Gale E, Smoll N, Al Imam MH, et al. Epidemiology of respiratory syncytial virus in Central Queensland, Australia. Commun Dis Intell 2024; 48: e-pub (https://doi.org/10.33321/cdi.2024.48.45).
37. Morley C, Grimwood K, Maloney S, Ware RS. Meteorological factors and respiratory syncytial virus seasonality in subtropical Australia. Epidemiol Infect 2018; 146: 757-762.
38. Hogan AB, Anderssen RS, Davis S, et al. Time series analysis of RSV and bronchiolitis seasonality in temperate and tropical Western Australia. Epidemics 2016; 16: 49-55.
39. Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis 2021; 72: 2199-2202.
40. El-Heneidy A, Ware RS, Robson JM, Cherian SG, Lambert SB, Grimwood K. Respiratory virus detection during the COVID-19 pandemic in Queensland, Australia. Aust N Z J Public Health 2022; 46: 10-15.
41. Leija-Martinez JJ, Esparza-Miranda LA, Rivera-Alfaro G, Noyola DE. Impact of nonpharmaceutical interventions during the COVID-19 pandemic on the prevalence of respiratory syncytial virus in hospitalized children with lower respiratory tract infections: a systematic review and meta-analysis. Viruses 2024; 16: 429.
42. Eden JS, Sikazwe C, Xie R, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun 2022; 13: 2884.
43. Burrell R, Saravanos G, Britton PN. Unintended impacts of COVID-19 on the epidemiology and burden of paediatric respiratory infections. Paediatr Respir Rev 2023 Aug 3; e-pub (http://dx.doi.org/10.1016/j.prrv.2023.07.004).
44. Walker GJ, Foster CSP, Sevendal A, et al. Clinical, genomic, and immunological characterization of RSV surge in Sydney, Australia, 2022. Pediatrics 2024; 153: e2023063667.
45. National Notifiable Diseases Surveillance System (NNDSS). National Communicable Disease Surveillance Dashboard. Canberra: Australian Government Department of Health and Aged Care; 2024. Accessed September 1, 2024. Available online at: https://nindss.health.gov.au/pbi-dashboard/ (accessed September 2024).
46. Australian Centre for Disease Control. Australian Respiratory Surveillance Report 11, 12 August to 25 August 2024. Canberra: Australian Government Department of Health and Aged Care; 2024. 29p. Available online at: https://www.health.gov.au/sites/default/files/2024-08/australian-respiratory-surveillance-report-10-12-august-to-25-august-2024.pdf (accessed September 2024).
47. Shi T, Vennard S, Mahdy S, Nair H; RESCEU investigators. Risk factors for poor outcome or death in young children with respiratory syncytial virus-associated acute lower respiratory tract infection: a systematic review and meta-analysis. J Infect Dis 2022; 226 Suppl 1: S10-S16.
48. Australian Immunisation Handbook. Respiratory syncytial virus (RSV). Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/respiratory-syncytial-virus-rsv (accessed September 2024).
49. National Health and Medical Research Council (NHMRC). Australian Guidelines for the Prevention and Control of Infection in Healthcare, v11.24. Canberra: NHMRC; 2024. Available online at: https://app.magicapp.org/#/guideline/Jn37kn (accessed September 2024).
50. Australian Commission on Safety and Quality in Health Care (ACSQHC). Alcohol-based hand rubs. Sydney: ACSQHC; 2024. Available online at: https://www.safetyandquality.gov.au/our-work/infection-prevention-and-control/national-hand-hygiene-initiative/what-hand-hygiene/alcohol-based-hand-rubs (accessed September 2024).
51. Therapeutic Goods Administration. SYNAGIS Palivizumab (rmc) 100 mg / 1 mL Solution for Injection Vial (231139). Australian Register of Therapeutic Goods. Canberra: Australian Government Department of Health and Aged Care; 2023. Available online at: https://www.tga.gov.au/resources/artg/231139 (accessed September 2024).
52. The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102: 531-537.
53. Anderson EJ, Carosone-Link P, Yogev R, Yi J, Simões EAF. Effectiveness of palivizumab in high-risk infants and children. Pediatr Infect Dis J 2017; 36: 699-704.
54. Therapeutic Goods Administration. Australian Public Assessment Report for Beyfortus. Canberra: Australian Government Department of Health and Aged Care; 2024. 38p. Available online at: https://www.tga.gov.au/sites/default/files/2024-04/auspar-beyfortus-240412.pdf (accessed September 2024).
55. Robbie GJ, Criste R, Dall’acqua WF, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 2013; 57: 6147-6153.
56. Griffin MP, Khan AA, Esser MT, et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother 2017; 61: e01714-16.
57. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386: 837-846.
58. McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340: 1113-1117.
59. Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9: eaaj1928.
60. Therapeutic Goods Administration. Australian Product Information – Beyfortus (Nirsevimab) Solution for Injection (Sanofi-Aventis Australia Pty Ltd). Canberra: Australian Government Department of Health and Aged Care; 2024. 19p. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2023-PI-02637-1&d=20240821172310101 (accessed September 2024).
61. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383: 415-425.
62. Domachowske J, Madhi SA, Simoes EAF, et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. N Engl J Med 2022; 386: 892-894.
63. Drysdale SB, Cathie K, Flamein F, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med 2023; 389: 2425-2435.
64. Paireau J, Durand C, Raimbault S, et al. Nirsevimab effectiveness against cases of respiratory syncytial virus bronchiolitis hospitalised in paediatric intensive care units in France, September 2023-January 2024. Influenza Other Respir Viruses 2024; 18: e13311.
65. Ernst C, Bejko D, Gaasch L, et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Euro Surveill 2024; 29: 2400033.
66. Ezpeleta G, Navascues A, Viguria N, et al. Effectiveness of nirsevimab immunoprophylaxis administered at birth to prevent infant hospitalisation for respiratory syncytial virus infection: a population-based cohort study. Vaccines (Basel) 2024; 12: 383.
67. Lopez-Lacort M, Munoz-Quiles C, Mira-Iglesias A, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Euro Surveill 2024;29: 2400046.
68. Assad Z, Romain AS, Aupiais C, et al. Nirsevimab and hospitalization for RSV bronchiolitis. N Engl J Med 2024; 391: 144-154.
69. Consolati A, Farinelli M, Serravalle P, et al. Safety and efficacy of nirsevimab in a universal prevention program of respiratory syncytial virus bronchiolitis in newborns and infants in the first year of life in the Valle d’Aosta Region, Italy, in the 2023-2024 epidemic season. Vaccines (Basel) 2024; 12: 549.
70. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus-associated hospitalization among infants entering their first respiratory syncytial virus season - new vaccine surveillance network, October 2023 - February 2024. MMWR Morb Mortal Wkly Rep 2024; 73: 209-214.
71. Ares-Gómez S, Mallah N, Santiago-Perez MI, et al. Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study. Lancet Infect Dis 2024; 24: 817-828.
72. Dagan R, Hammitt LL, Seoane Nunez B, et al. Infants receiving a single dose of nirsevimab to prevent RSV do not have evidence of enhanced disease in their second RSV season. J Pediatric Infect Dis Soc 2024; 13: 144-147.
73. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices – United States, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 920-925.
74. American Academy of Pediatrics (AAP). AAP Recommendations for the Prevention of RSV Disease in Infants and Children. Itasca, IL: AAP, Red Book Online; 2024. Available online at: https://publications.aap.org/redbook/resources/25379/AAP-Recommendations-for-the-Prevention-of-RSV (accessed September 2024).
75. Government of Western Australia Department of Health. Respiratory syncytial virus (RSV) immunisation. Perth: Government of Western Australia Department of Health ; 2024. Available online at: https://www.health.wa.gov.au/Articles/N_R/Respiratory-syncytial-virus-RSV-immunisation (accessed September 2024).
76. Queensland Health. Queensland paediatric respiratory syncytial virus prevention program. Brisbane: Queensland Health; 2024. Available online at: https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/ diseases-infection/immunisation/paediatric-rsv-prevention-program (accessed September 2024).
77. NSW Ministry of Health. NSW Health RSV (Respiratory Syncytial Virus) vulnerable babies program. Sydney: NSW Health; 2024. Available online at: https://www.health.nsw.gov.au/immunisation/Pages/respiratory-syncytial-virus.aspx (accessed September 2024).
78. Pharmaceutical Benefits Advisory Committee. Pharmaceutical Benefits Advisory Committee (PBAC) Meeting Outcomes – July 2024 PBAC Meeting. Canberra: Australian Government Department of Health and Aged Care; 2024. 35p. Available online at: https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2024-07/pbac-web-outcomes-07-2024.pdf (accessed September 2024).
79. Therapeutic Goods Administration. Australian Public Assessment Report for Arexvy. Canberra: Australian Government Department of Health and Aged Care; 2024. 34p. Available online at: https://www.tga.gov.au/sites/default/files/2024-05/auspar-arexvy-240503.pdf (accessed September 2024).
80. Therapeutic Goods Administration. Australian Public Assessment Report for Abrysvo. Canberra: Australian Government Department of Health and Aged Care; 2024. 59p. Available online at: https://www.tga.gov.au/sites/default/files/2024-05/auspar-abrysvo-240502.pdf (accessed September 2024).
81. Ruckwardt TJ. The road to approved vaccines for respiratory syncytial virus. NPJ Vaccines 2023; 8: 138.
82. Leroux-Roels I, Davis MG, Steenackers K, et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F (RSVPreF3) candidate vaccine in older adults: phase 1/2 randomized clinical trial. J Infect Dis 2023; 227: 761-772.
83. Therapeutic Goods Administration. Australian Product Information – Arexvy (Recombinant Respiratory Syncytial Virus Pre-Fusion F Protein) Powder and Suspension for Injection (GlaxoSmithKline Australia Pty Ltd). Canberra: Australian Government Department of Health and Aged Care; 2024. 17p. Available online at: https://www.tga.gov.au/sites/default/files/2024-05/auspar-arexvy-240503-pi.pdf (accessed September 2024).
84. Therapeutic Goods Administration. Australian Product Information – Abrysvo (Recombinant Respiratory Syncytial Virus Pre-Fusion F Protein) Vaccine (Pfizer Australia Pty Ltd). Canberra: Australian Government Department of Health and Aged Care; 2024. 30p. Available online at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2024-PI-01489-1&d=20240528172310101 (accessed September 2024).
85. Pharmaceutical Benefits Advisory Committee. Pharmaceutical Benefits Advisory Committee (PBAC) Meeting Outcomes – May 2024 PBAC Meeting. Canberra: Australian Government Department of Health and Aged Care; 2024. 8p. Available online at: https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2024-05/pbac-web-outcomes-05-2024.pdf (accessed September 2024).
86. Pharmaceutical Benefits Advisory Committee. Pharmaceutical Benefits Advisory Committee (PBAC) Meeting Agenda – November 2024 PBAC Meeting – Version 3. Canberra: Australian Government Department of Health and Aged Care; 2024. 32p. Available online at: https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/agenda/pdf/2024/PBAC-meeting-agenda-November-2024-v3.pdf (accessed September 2024).
87. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388: 595-608.
88. Ison MG, Papi A, Athan E, et al. Efficacy and safety of respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons. Clin Infect Dis 2024; 78: 1732-1744.
89. Walsh EE, Perez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388: 1465-1477.
90. Pfizer. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO for RSV in older adults. Press release. New York, NY: Pfizer; 2024. Available online at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two (accessed September 2024).
91. Hutton DW. Risk-benefit analysis of RSV vaccination in older adults. Paper presented at: Meeting of the Advisory Committee On Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC), 2024 June 26-28; Atlanta, GA. Available online at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-06-26-28/09-RSV-Adult-Hutton-508.pdf (accessed September 2024).
92. Hause AM, Moro PL, Baggs J, et al. Early safety findings among persons aged ≥60 years who received a respiratory syncytial virus vaccine - United States, May 3, 2023 - April 14, 2024. MMWR Morb Mortal Wkly Rep 2024; 73: 489-494.
93. Lloyd P. Evaluation of Guillain-Barré Syndrome (GBS) following respiratory syncytial virus (RSV) vaccination among adults 65 years and older. Paper presented at: Meeting of the Advisory Committee On Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC), 2024 June 26-28; Atlanta, GA. Available online at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-06-26-28/06-RSV-Adult-Lloyd-508.pdf (accessed September 2024).
94. Dieussaert I, Hyung Kim J, Luik S, et al. RSV prefusion F protein-based maternal vaccine - preterm birth and other outcomes. N Engl J Med 2024; 390: 1009-1021.
95. Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med 2023; 388: 1451-1464.
96. Son M, Riley LE, Staniczenko AP, et al. Nonadjuvanted bivalent respiratory syncytial virus vaccination and perinatal outcomes. JAMA Netw Open 2024; 7: e2419268.
97. Athan E, Baber J, Quan K, et al. Safety and immunogenicity of bivalent RSVpreF vaccine coadministered with seasonal inactivated influenza vaccine in older adults. Clin Infect Dis 2024; 78: 1360-1368.
98. Chandler R, Montenegro N, Llorach C, et al. Immunogenicity, reactogenicity, and safety of AS01E-adjuvanted respiratory syncytial virus (RSV) prefusion F protein-based candidate vaccine (RSVPreF3 OA) when co-administered with a seasonal quadrivalent influenza vaccine in older adults: results of a phase 3, open-label, randomized controlled trial. Clin Infect Dis 2024 Jan 8; e-pub (http://dx.doi.org/10.1093/cid/ciad786).
99. Willemsen JE, Borghans JAM, Bont LJ, Drylewicz J. Maternal vaccination against RSV can substantially reduce childhood mortality in low-income and middle-income countries: A mathematical modeling study. Vaccine X 2023; 15: 100379.
100. Wilkins D, Yuan Y, Chang Y, et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat Med 2023; 29: 1172-1179.
101. Mullard A. FDA approves mRNA-based RSV vaccine. Nat Rev Drug Discov 2024; 23: 487.
102. Wilson E, Goswami J, Baqui AH, et al. Efficacy and safety of an mRNA-based RSV preF vaccine in older adults. N Engl J Med 2023; 389: 2233-2244.
103. Shaw CA, Mithani R, Kapoor A, et al. Safety, tolerability and immunogenicity of a mRNA-based RSV vaccine in healthy young adults in a phase 1 clinical trial. J Infect Dis 2024 Jan 31; e-pub (http://dx.doi.org/10.1093/infdis/jiae035).
104. Goswami J, Baqui AH, Doreski PA, et al. Humoral immunogenicity of mRNA-1345 RSV vaccine in older adults. J Infect Dis 2024 Jun 18; e-pub (http://dx.doi.org/10.1093/infdis/jiae316).
105. Sanchez-Martinez ZV, Alpuche-Lazcano SP, Stuible M, Durocher Y. CHO cells for virus-like particle and subunit vaccine manufacturing. Vaccine 2024; 42: 2530-2542.
106. Mazur NI, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis 2023; 23: e2-e21.
107. Cockerill GS, Angell RM, Bedernjak A, et al. Discovery of sisunatovir (RV521), an inhibitor of respiratory syncytial virus fusion. J Med Chem 2021; 64: 3658-3676.
108. Nilsson AC, Pullman J, Napora P, et al. A pilot phase 2a, randomized, double-blind, placebo-controlled study to explore the antiviral activity, clinical outcomes, safety, and tolerability of rilematovir at two dose levels in non-hospitalized adults with respiratory syncytial virus infection. Clin Microbiol Infect 2023; 29: 1320-1327.
109. Ferrero F, Lin CY, Liese J, et al. CROCuS, a Phase II study evaluating the antiviral activity, clinical outcomes, and safety of rilematovir in children aged ≥ 28 days and ≤ 3 years with acute respiratory tract infection due to respiratory syncytial virus. Paediatr Drugs 2024; 26: 411-427.
110. Pharmaceutical Benefits Advisory Committee. Pharmaceutical Benefits Advisory Committee (PBAC) Meeting Outcomes – March 2024 PBAC Meeting. Canberra: Australian Government Department of Health and Aged Care; 2024. 57p. Available online at: https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/recommendations-made-by-the-pbac-march-2024 (accessed September 2024).
111. Peterson JT, Zareba AM, Fitz-Patrick D, et al. Safety and immunogenicity of a respiratory syncytial virus prefusion F vaccine when coadministered with a tetanus, diphtheria, and acellular pertussis vaccine. J Infect Dis 2022; 225: 2077-2086.
112. Shearer FM, Edwards L, Kirk M, et al. Opportunities to strengthen respiratory virus surveillance systems in Australia: lessons learned from the COVID-19 response. Commun Dis Intell. 2024; 48: e-pub (https://doi.org/10.33321/cdi.2024.48.47).