Drug-induced interstitial lung disease – a heterogeneous old foe with an evolving new face
Drug-induced interstitial lung disease (DIILD) comprises a diverse group of conditions caused by exposure to specific medications. Its diagnosis depends on high clinical suspicion and the exclusion of differential diagnoses. Management of DIILD largely involves cessation of the inciting medication, along with the use of systemic corticosteroids or other immunosuppressants in severe cases.
- Drug-induced interstitial lung disease (DIILD) is a highly heterogenous condition.
- The true incidence of DIILD is unknown but will likely increase in the future.
- The diagnosis of DIILD is one of exclusion, which can be challenging in medically complex patients. High-resolution CT is the investigation of choice.
- Cessation of the inciting medication is the primary strategy for management.
- All patients with DIILD must undergo follow up with the prescribing specialist. Patients with significant or difficult- to-control disease, or an uncertain diagnosis, need to be referred to a respiratory physician for assessment.
- Systemic corticosteroids or other forms of immuno- suppression may be indicated in certain cases following consultations with prescribing specialists and respiratory physicians.
Drug-induced interstitial lung disease (DIILD) comprises a group of highly heterogeneous conditions that encompass clinical sequelae of lung injury and inflammation with or without resultant fibrosis following exposure to a specific medication. With the rapid growth in pharmaceutical research, the natural history and presentations of DIILD encountered in clinical practice continue to evolve in parallel with the discovery and expansion of novel and existing drug classes. Along with the unpredictable nature of DIILD, this evolution poses challenges for GPs who are at the forefront of patient care, requiring a high index of suspicion and awareness for timely assessment and management.
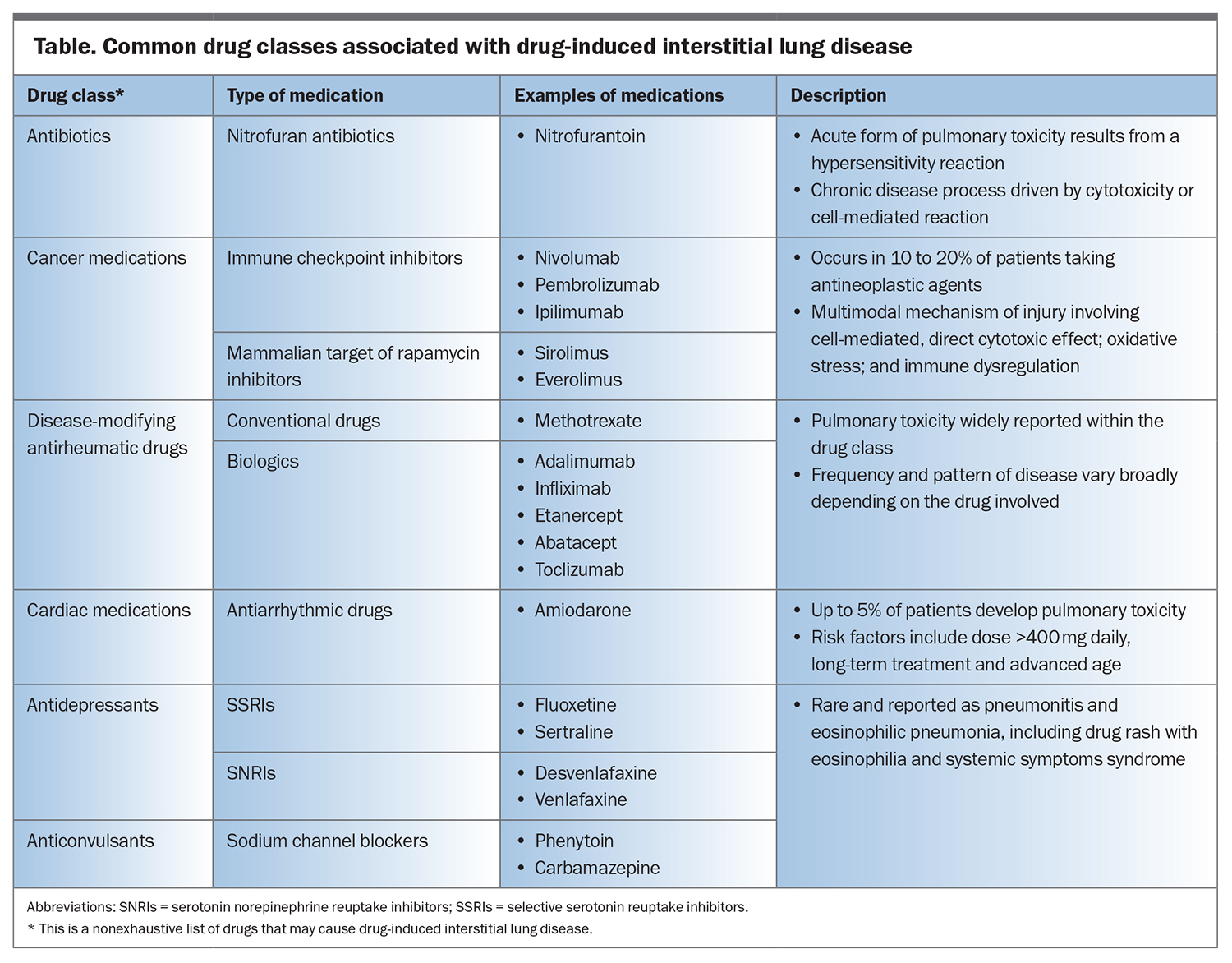
Our understanding of DIILD remains limited, with most knowledge being derived from postmarketing surveillance studies.1 The actual incidence of DIILD is unknown and varies significantly depending on the patient demographic, drug type and dose involved and data source.2 The incidence of DIILD is estimated to range from 4.1 to 12.4 cases per million people annually.1 However, this estimation does not include cases involving drug classes recently established to cause DIILD, such as immune checkpoint inhibitors used for cancer treatment and biologics used to treat rheumatological conditions, and hence may not capture the full spectrum of DIILD. Key common medications that are known to cause DIILD are listed in the Table, which is nonexhaustive. An ever-expanding list of hundreds of drugs are associated with lung toxicity, which is continually updated on Pneumotox (http://www.pneumotox.com), a free and searchable web-based database available as a reference for clinical use. This article provides an overview of DIILD, highlighting the various mechanisms, evolving nature of its clinical presentations, challenges in diagnosis, management approaches and the need for heightened awareness among GPs.
Pathophysiology and mechanisms of DIILD
Although the exact mechanisms of drug-induced lung injury and associated underlying pathogenesis of DIILD are not well understood, the disease is postulated to be primarily related to direct cytotoxic injury or dysregulated immune-mediated damage (Table).2 In cases of severe lung injury and uncontrolled inflammation, fibrosis and lung scarring can ensue.
Direct cytotoxic lung injury
Within the lungs, pneumocytes and the alveolar capillary endothelium are susceptible to cytotoxic damage, which can occur via a myriad of mechanisms, such as the drug itself and its production of toxic metabolites. Amiodarone, a common antiarrhythmic agent, is a classic example of a drug causing direct cytotoxic lung injury. The injury occurs through several pathways by promoting the release of oxygen free radicals and activating downstream proteolytic enzymes, causing lung epithelial cell death, as well as deactivating processes that clear its toxic metabolites.3-5 Local inflammation at the injury sites may increase capillary permeability, thus causing pulmonary oedema.
Dysregulated immune-mediated lung damage
The lung parenchyma harnesses components of both innate and adaptive immune responses to protect against infection and environmental irritants.6 Exposure to a specific medication can dysregulate this immune protective barrier and lead to an inflammatory cascade, resulting in lung parenchymal damage.5,7 Immune checkpoint inhibitors, the latest advances in cancer treatment, act by improving the ability of the body to recognise and target cancer cells, largely through priming T-lymphocytes. Such interferences can cause the immune system to attack noncancer cells within the lung interstitium that share molecular targets, leading to pneumonitis.8 Patients receiving a combination of different immune checkpoint inhibitors or who have pre-existing interstitial lung disease are at an increased risk of developing pneumonitis.8,9
Other mechanisms
Methotrexate-induced lung injury is a key consideration in patients who experience the acute onset or worsening of breathlessness while receiving methotrexate for a variety of rheumatological conditions, most commonly rheumatoid arthritis. The mechanism of injury is not clear, although it is thought to involve a lymphocyte-driven hypersensitivity reaction. Risk factors for methotrexate-induced lung injury include advanced age (>60 years), pre-existing lung disease and diabetes mellitus.2 Of note, the role of methotrexate in long-term pulmonary fibrosis has come into question, with recent emergent data from multiple studies showing low incidences of pulmonary fibrosis and reduced cases of interstitial lung disease within methotrexate-treated patient populations with rheumatoid arthritis.2
In rare instances, DIILD may manifest as pulmonary infiltrates and peripheral eosinophilia, which can occur following exposure to antibiotics, anticonvulsants and antidepressants.2,10 In such cases, the disease process is mediated by the action of T-helper 2 lymphocytes through the release of inflammatory chemokines, and may result in a severe idiosyncratic drug reaction characterised by extensive skin rash and various organ involvement, known as drug rash with eosinophilia and systemic symptoms syndrome.11,12 Impairment of the protective mechanisms responsible for alveolar repair can propagate drug-induced damage, which is seen with gefitinib, a cancer treatment that inhibits epidermal growth factor receptor signalling.13
Diagnosis and management of DIILD
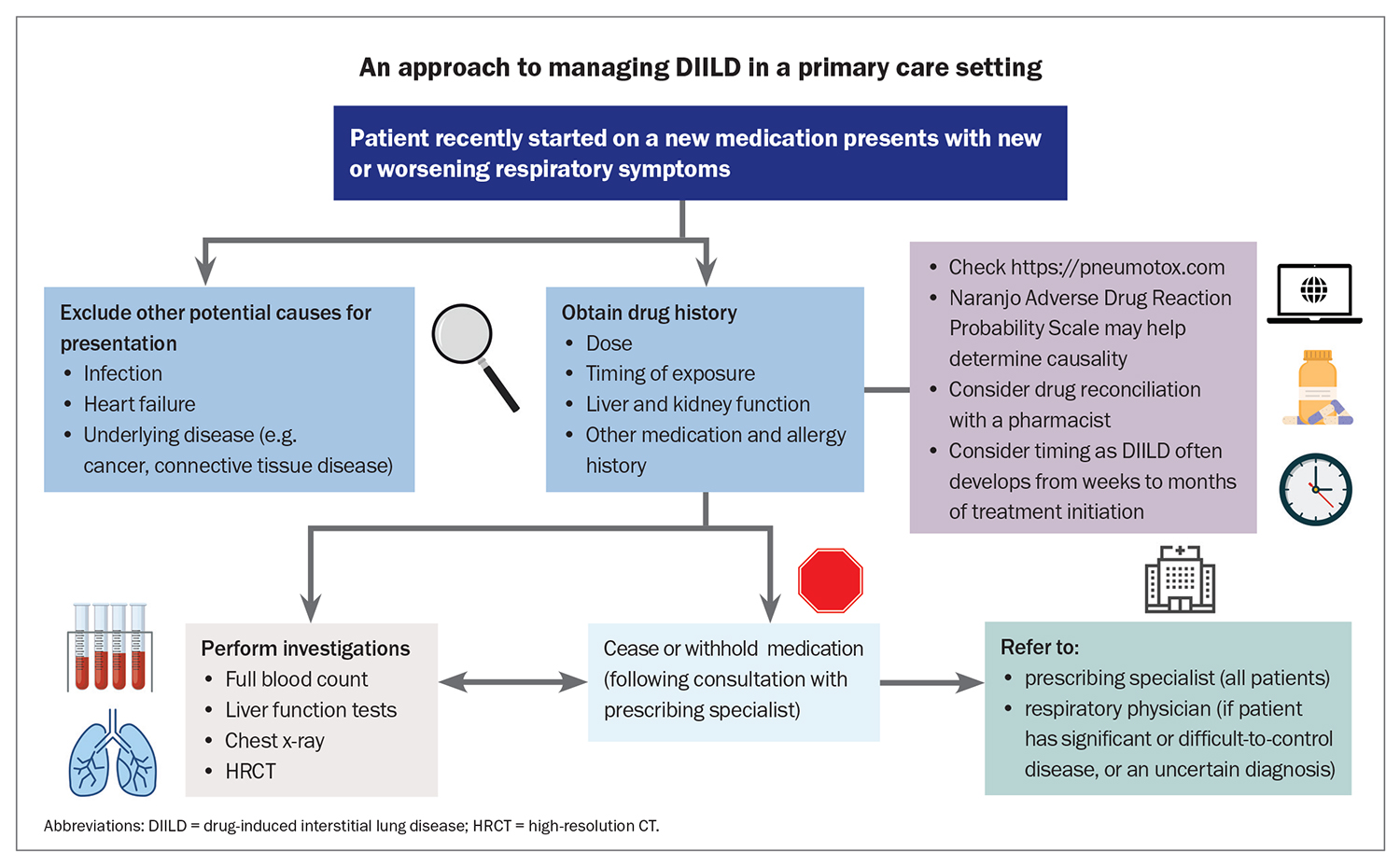
Effective management and the prevention of irreversible damage from DIILD are centred around early detection. The diagnosis of DIILD is one of exclusion and relies on multimodal clinical, laboratory and imaging assessments in the context of temporal exposure to a specific medication. As DIILD can present across a broad spectrum of severity, ranging from an incidental finding to fulminant respiratory failure, the diagnostic and management approaches vary significantly. Before arriving at a diagnosis, clinicians must consider several differential diagnoses, as DIILD can mimic many common conditions in a medically complex case. Although ceasing the causative medication is sufficient in most cases but systemic corticosteroids and other immunomodulatory therapies may be required in more severe cases. The Flowchart illustrates a practical approach for managing DIILD in a primary care setting.
Clinical assessments
A detailed initial assessment is key to arriving at a diagnosis of DIILD, which is highly dependent on an accurate drug history that incites clinical suspicion. Clinical presentations of DIILD are typically characterised by breathlessness and reduced exercise tolerance over a subacute time course of weeks to months, although some patients may have acute or chronic disease courses. Acute presentations of DIILD can be associated with constitutional symptoms, such as fever for acute presentations, whereas chronic presentations are typical of chronic lung disease.2 Such presentations can pose a challenge, as they can be attributed to a wide range of differential diagnoses and, thus, the initial work-up should focus on ruling out common or life-threatening diseases, such as infection, heart failure, cancer and other forms of interstitial lung disease. Lung auscultation may reveal fine ‘velcro-like’ crackles or features of fluid overload due to right ventricular dysfunction or pulmonary hypertension in advanced cases.
It is important to consider specific patient characteristics and the involved drugs when considering the likelihood of the diagnosis of DIILD. Established risk factors for DIILD include advanced age and current smoking status.1,2 The male sex is an additional risk factor for DIILD secondary to the use of epidermal growth factor receptor inhibitors, methotrexate and amiodarone.1,2 Nitrofurantoin-induced lung injury is more commonly seen in women than in men, given their increased risk of urinary tract infections, for which the medication is used as a preventative measure.14 Pre-existing lung conditions, including chronic obstructive pulmonary diseases and other interstitial lung diseases, and indicators of poor prognosis, such as poor performance status, have also been raised as independent risk factors for the development of DIILD.2
When taking a drug history, detailed information on the medication types, doses and timing should be determined. The risk of developing DIILD is dose-dependent with certain medications, such as bleomycin, amiodarone, nitrofurantoin, epidermal growth factor receptor inhibitors and gemcitabine.1 Medications that can cause severe DIILD requiring hospitalisation and treatment with systemic corticosteroids include epidermal growth factor receptor inhibitors and class III antiarrhythmic drugs (e.g. amiodarone).15
However, even with high-risk patient and drug profiles, determining the causality of a specific medication in DIILD remains challenging. Resources such as the Pneumotox website or mobile application and Naranjo Adverse Drug Reaction Probability Scale may be used as part of the clinical assessment.Pneumotox is a freely accessible repository built from bibliographic references with extensive information on drugs that can be associated with lung pathology. The Naranjo Adverse Drug Reaction Probability Scale is a 10-question tool that stratifies the likelihood of a clinical presentation being associated with medication exposure.16 Using such tools, in addition to considering an individual risk profile, may help ascertain the clinical suspicion of DIILD and prompt further investigation.
Investigations
Investigations for clinical presentations of possible cases of DIILD include tests to confirm or strengthen a suspicion and rule out conditions that may mimic DIILD following clinical assessments.
Blood tests
Routine laboratory blood tests may point to an infection or heart failure. Measuring the serum level of pro B-type natriuretic peptide can serve as a quick avenue to either dispel or confirm a suspicion of heart failure, prompting referral for echocardiography and the involvement of a cardiologist. Reduced drug clearance and the risk of drug accumulation may be evaluated with renal and hepatic function blood tests, which are important in determining the safety and appropriateness of drug dosing. If there are concerns of an alternative diagnosis of interstitial lung disease, such as connective tissue disease, serology testing for autoantibodies can be performed with the consideration of referral to a respiratory physician.17,18
Chest imaging
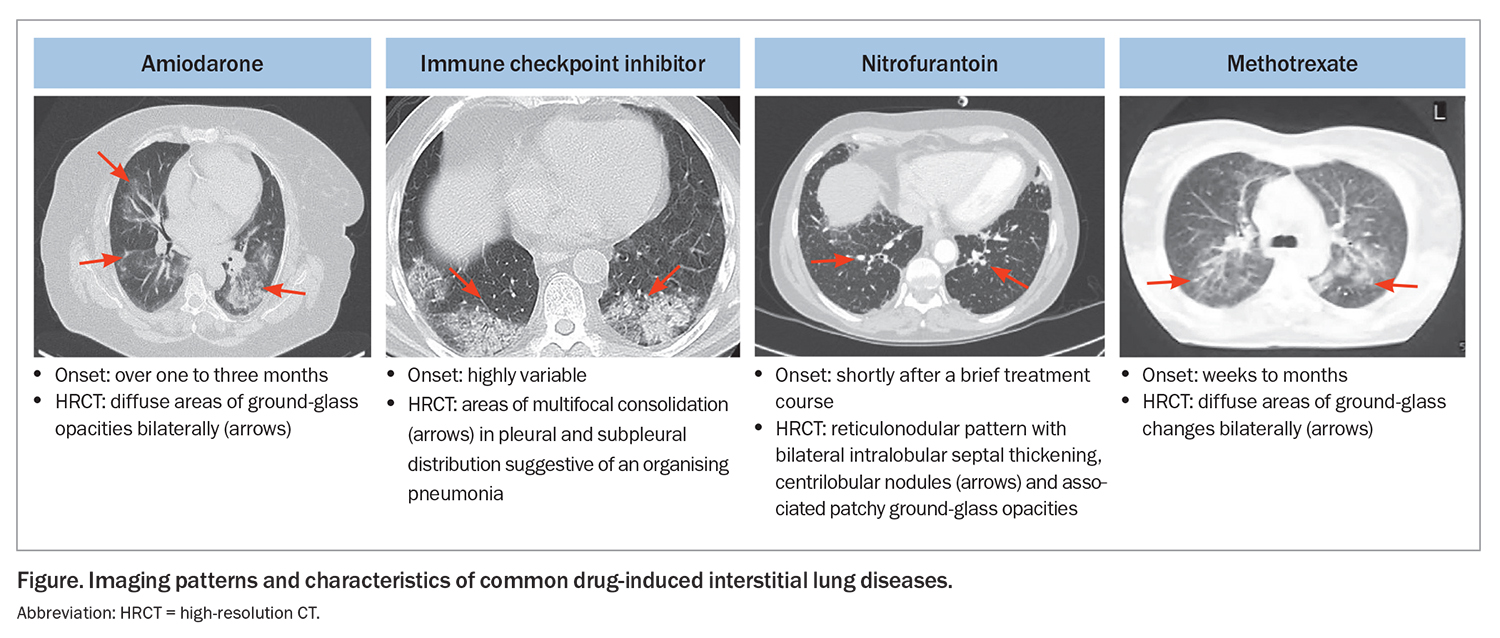
Imaging plays a central role in the work-up of DIILD. Although 25 to 75% of chest x-ray findings are unremarkable in cases of clinically suspected DIILD, x-rays still retain some utility, as they are quick to access and can help screen for many common disease processes, such as pneumonia and heart failure.19,20 High-resolution CT is the modality of choice for detecting DIILD and can be helpful in cases where the aetiology of the presentation is unclear. Common findings on high-resolution CT for DIILD include ground-glass opacity, interlobular septal thickening, consolidation and centrilobular nodules (Figure).21 The Fleischner Society, an international multidisciplinary society for thoracic radiology dedicated to the diagnosis and treatment of diseases of the chest, has released a position paper on diagnostic criteria for DIILD secondary to molecular-targeted therapies and immune checkpoint inhibitors for cancers, which include:
- the development of parenchymal opacities bilaterally in a nonsegmental distribution observed on CT
- imaging features that exclude other interstitial lung diseases
- temporal association with exposure to medicine.22
Importantly, the radiological changes associated with DIILD have a high degree of overlap with those associated with other diagnoses of interstitial lung disease and common clinical presentations, including infection, and cannot be used solely to diagnose DIILD. Thus, these changes must be interpreted in the context of clinical assessments and other investigations. Multidisciplinary team discussions involving respiratory physicians, chest radiologists and pathologists, and other specialists are sometimes needed to establish a diagnosis. Additional invasive investigations, such as bronchoscopy and lung biopsy, may be performed after specialist assessment.
Management
Management in most cases of DIILD involves ceasing the inciting medication and avoiding future exposure to prevent permanent lung injury. As the diagnosis of DIILD can be delayed, while awaiting the results of investigations and specialist opinion, the medication of concern should be ceased promptly in cases where there is either strong clinical suspicion or severe respiratory compromise. If a diagnosis of DIILD is uncertain in the presence mild clinical symptoms, the medication should be ceased following a discussion and follow up with the prescribing specialist. In selected clinical situations, a cautious rechallenge with close monitoring by the specialist may be considered following resolution of the initial episode of DIILD and adequate patient counselling and discussion. For example, in patients with metastatic cancer who develop immune checkpoint inhibitor-induced pneumonitis, continuing the treatment may outweigh the risk of recurrent DIILD.
Patients with severe, persistent or recurring clinical and imaging abnormalities after ceasing the inciting medication, as well as those with an uncertain diagnosis, must be referred to a respiratory specialist. If ceasing the medication fails to stop disease progression, the addition of systemic corticosteroids may be needed.2 The dosage and duration vary and rely on the type of the inciting medication, imaging characteristics observed on CT and severity and acuity of clinical presentations. Systemic corticosteroids are frequently administered in a gradual, tapering regimen over a few weeks. Some patients may experience recurrence following withdrawal or weaning of the initial course of systemic corticosteroids and may require treatment with additional immunomodulatory medications and for ongoing monitoring.
Conclusion
DIILD is a significant presentation in clinical practice that requires high clinical suspicion. Although its exact incidence is not known, cases of DIILD will likely increase in the future with rapid advancements in drug development, particularly in the fields of oncology and rheumatology. It is essential to remain vigilant of the potential risks of DIILD associated with medications such as amiodarone and nitrofurantoin, given that they are widely used in the community. The cornerstone of management for DIILD centres on early detection and the timely cessation of the inciting medication, which follows expedited and comprehensive evaluation that includes a thorough medication history. Involvement of the prescribing specialist is necessary for ongoing treatment decisions, and a referral to a respiratory physician should be considered, particularly in severe and recurrent cases of DIILD. RMT
COMPETING INTERESTS: Dr Shivakumar: None. Associate Professor Khor has received grants from the National Health and Medical Research Council, Medical Research Future Fund, Austin Medical Research Foundation, Lung Foundation Australia/ Thoracic Society of Australia and New Zealand and Royal Australasian College of Physicians; has received in-kind trial support from Air Liquide Healthcare; is Board Director and Special Interest Group Convenor at the Thoracic Society of Australia and New Zealand; is a Clinical Problems Assembly Program Committee member and Guideline Methodologist at the American Thoracic Society; and is Associate Editor for the European Respiratory Journal by the European Respiratory Society.
References
1. Skeoch S, Weatherley N, Swift AJ, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med 2018; 7: 356.
2. Spagnolo P, Bonniaud P, Rossi G, Sverzellati N, Cottin V. Drug-induced interstitial lung disease. Eur Respir J 2022; 60: 2102776.
3. Pietra GG. Pathologic mechanisms of drug-induced lung disorders. J Thorac Imaging 1991; 6: 1-7.
4. Gram TE. Chemically reactive intermediates and pulmonary xenobiotic toxicity. Pharmacol Rev 1997; 49: 297-341.
5. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 2012; 13: 39.
6. Gruchalla RS. Drug metabolism, danger signals, and drug-induced hypersensitivity. J Allergy Clin Immunol 2001; 108: 475-488.
7. Matzinger P. The danger model: a renewed sense of self. Science 2002; 296: 301-305.
8. Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev 2019; 28: 190012.
9. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022; 19: 254-267.
10. Rizos E, Tsigkaropoulou E, Lambrou P, et al. Risperidone-induced acute eosinophilic pneumonia. In Vivo 2013; 27: 651-653.
11. Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia: a review of 196 case reports. Medicine (Baltimore) 2018; 97: e9688.
12. Choudhary S, McLeod M, Torchia D, Romanelli P. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Clin Aesthet Dermatol 2013; 6: 31-37.
13. Takano T, Ohe Y, Kusumoto M, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer 2004; 45: 93-104.
14. Santos JM, Batech M, Pelter MA, Deamer RL.Evaluation of the risk of nitrofurantoin lung injury and its efficacy in diminished kidney function in older adults in a large integrated healthcare system: a matched cohort study. J Am Geriatr Soc 2016; 64: 798-805.
15. Jo T, Michihata N, Yamana H, et al. Risk of drug-induced interstitial lung disease in hospitalised patients: a nested case-control study. Thorax 2021; 76: 1193-1199.
16. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239-245.
17. Prasad JD, Mahar A, Bleasel J, et al. The interstitial lung disease multidisciplinary meeting: a position statement from the Thoracic Society of Australia and New Zealand and the Lung Foundation Australia. Respirology 2017; 22: 1459-1472.
18. Jee AS, Sheehy R, Hopkins P, et al. Diagnosis and management of connective tissue disease-associated interstitial lung disease in Australia and New Zealand: a position statement from the Thoracic Society of Australia and New Zealand. Respirology 2021; 26: 23-51.
19. Chap L, Shpiner R, Levine M, Norton L, Lill M, Glaspy J. Pulmonary toxicity of high-dose chemotherapy for breast cancer: a non-invasive approach to diagnosis and treatment. Bone Marrow Transplant 1997; 20: 1063-1067.
20. Padley SP, Adler B, Hansell DM, Müller NL. High-resolution computed tomography of drug-induced lung disease. Clin Radiol 1992; 46: 232-236.
21. Cleverley JR, Screaton NJ, Hiorns MP, Flint JD, Müller NL. Drug-induced lung disease: high-resolution CT and histological findings. Clin Radiol 2002; 57: 292-299.
22. Johkoh T, Lee KS, Nishino M, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Chest 2021; 159: 1107-1125.