Cystic fibrosis management: successes and challenges
Outcomes for people with cystic fibrosis (CF) have dramatically improved over the past few decades, particularly with the development of small-molecule cystic fibrosis transmembrane conductance regulator modulators, which specifically target the disease process. A child born with CF today can potentially expect to enjoy a normal life expectancy, albeit one that requires ongoing medical support.
- Cystic fibrosis (CF), the most common life-limiting genetic mutation, is caused by genetic defects on chromosome 7 that affect the cystic fibrosis transmembrane conductance regulator (CFTR), a chloride ion transport protein.
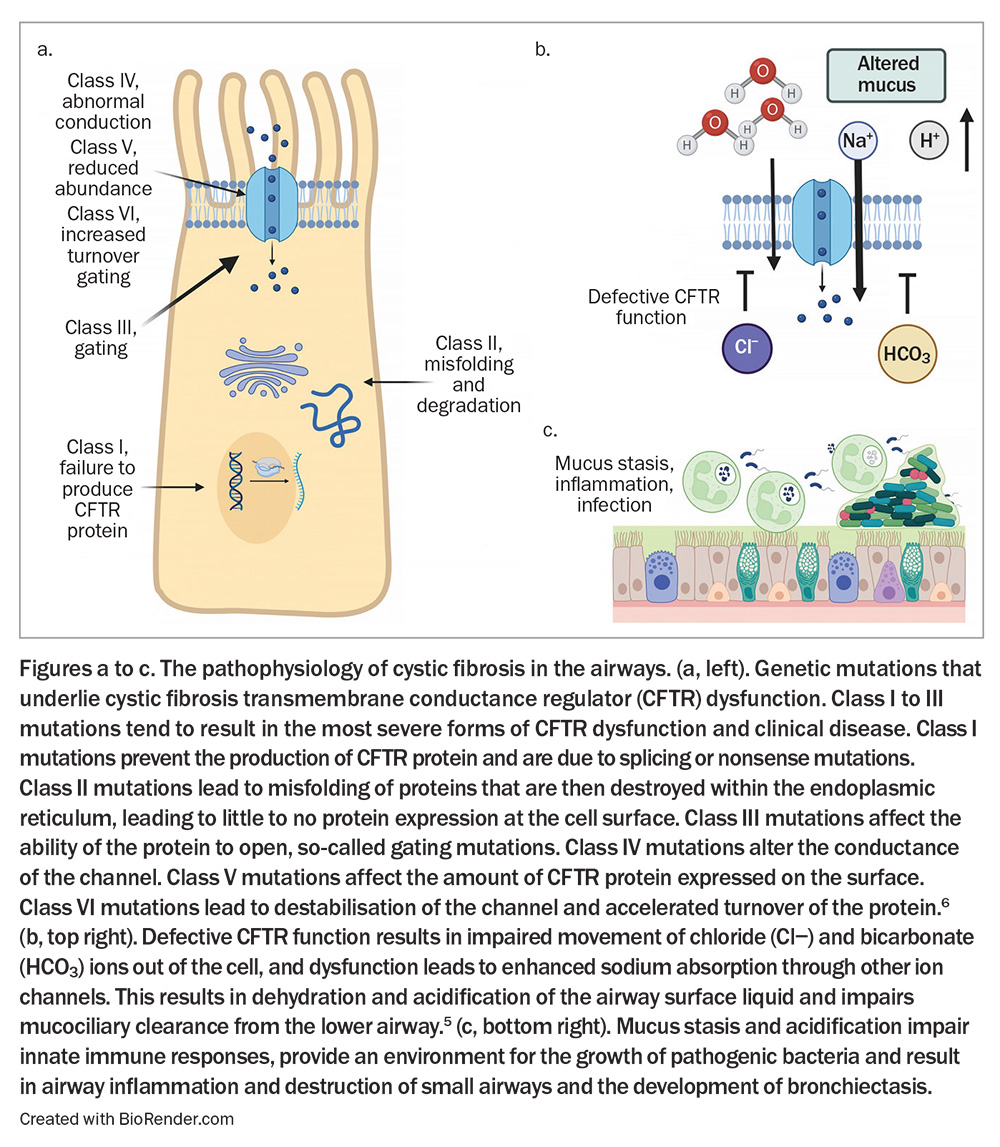
- Dysfunction of the CFTR protein results in enhanced sodium absorption and reduced release of bicarbonate, which, in the airway, leads to dehydration and acidification of the airway surface liquid and, subsequently, to abnormal mucus and impaired mucociliary clearance. This encourages colonisation of the airway with bacterial pathogens.
- The advent in the past decade of small-molecule CFTR modulators, which enhance CFTR function directly and specifically target the disease process, promises to be transformational for people with CF. Their approval for use in ever younger populations who have not yet developed serious lung disease is expected to result in increased life expectancy for affected individuals.
- Despite the advances in treatment, challenges remain for people with CF. This includes increasing numbers of people with CF who will experience other health concerns and require support from a broader range of health professionals who until now have had little exposure to people with this disease.
- For those people in whom CFTR modulators are not available or intolerance precludes their use, alternative treatments should be sought and innovative approaches to clinical trial design considered to find a solution for everyone with this disease.
Cystic fibrosis (CF) is the most common life-limiting genetic mutation with an incidence of about one in 3000 people worldwide and affecting 3700 people in Australia.1 It affects about one in 2500 people of European background, but is increasingly recognised to be present, albeit in reduced prevalence, in most populations and should not be dismissed as a diagnosis based on ethnicity alone.2
CF results in a multisystem disease that leads to recurring chest infections with progressive loss of lung function and damage to the intestinal tract, pancreas and liver.3 These effects impose on the individual a need for lifelong specialist care, which is among the most intensive and costly of any disease. Notwithstanding this, the recent story of CF care is a remarkable one. It is a unique example of how the community and health professionals have worked together over many years in close collaboration to foster research that has uncovered much about the pathophysiology of the disease, organised effective responses to medical care and now shepherded in highly effective disease-specific treatment.
A rare genetic disorder affecting chloride ion conduction
CF is caused by genetic defects on chromosome 7 that affect the cystic fibrosis transmembrane conductance regulator (CFTR), a chloride ion transport protein.4 The CFTR protein is expressed on the surface of epithelial cells and is responsible for the movement of chloride and bicarbonate ions. Dysfunction of the CFTR protein results in enhanced sodium absorption and reduced release of bicarbonate, which, in the airway, leads to dehydration and acidification of the airway surface liquid.5 This then leads to abnormal mucus and impaired mucociliary clearance, which encourages colonisation of the airway with bacterial pathogens, provoking a cycle of recurring infection and inflammation, resulting in progressive lung damage – the primary cause of morbidity and mortality in CF (Figure).5,6 There are more than 350 mutations that are known to cause disease and a further 2000 genetic mutations in the CFTR gene that may contribute to dysfunction.7
Adding to the complexity is the impact of CFTR modifier genes and CFTR epigenetics, as well as the influence of environmental factors that combine to determine the final expression of the disease in an individual.8,9 Mutations in CFTR are classified into six broad categories that are helpful in understanding current approaches to managing CF (Figure).6
Although lung disease results in the major complications of CF, CFTR is expressed throughout the body, and, therefore, it is not only the lungs that are affected. People with CF develop early damage to the pancreas, resulting in exocrine failure in early childhood. This leads to fat and nutrient malabsorption, as well as abnormal gastrointestinal mucus, which results in constipation and, at times, bowel obstruction, the development of liver disease that appears in adolescence and the risk of diabetes that affects at least one-third of individuals by adulthood.10
Improved clinical outcomes for people with CF
Outcomes for people with CF have dramatically improved since CF was first described as a disease of unexplained malnutrition and death during infancy in 1938.11 Even before there were specific treatments that improved CFTR function, a model of care was developed that uniquely brought together multiple practitioners in a multidisciplinary team that was proactive, normalising nutrition at a young age with pancreatic enzyme-replacement therapy and dietetics input, implementing early introduction of airway clearance to reduce pulmonary infections and airway damage, aggressively treating infections when they occurred as well as eradicating organisms such as Pseudomonas when they were first detected, all of which became the standard of care in specialised CF centres.12
This model of care was consolidated further with the early detection of disease through newborn screening programs, the development of co-ordinated approaches to the disease through patient registries, clinical trials networks and partnering with patient organisations.12 Patient advocacy and philanthropy, along with partnerships between healthcare professionals and consumer groups, had a pivotal role, enabling trials to occur and medications to become approved in a timely way.13 As a result and before the approval of the first CFTR modulator in 2012 the median survival for a child born with CF had reached 40 years. This has continued to increase with the median survival now up to 58 years, and most people living with CF are now aged older than 18 years.1,14
CFTR modulators and their impact on disease
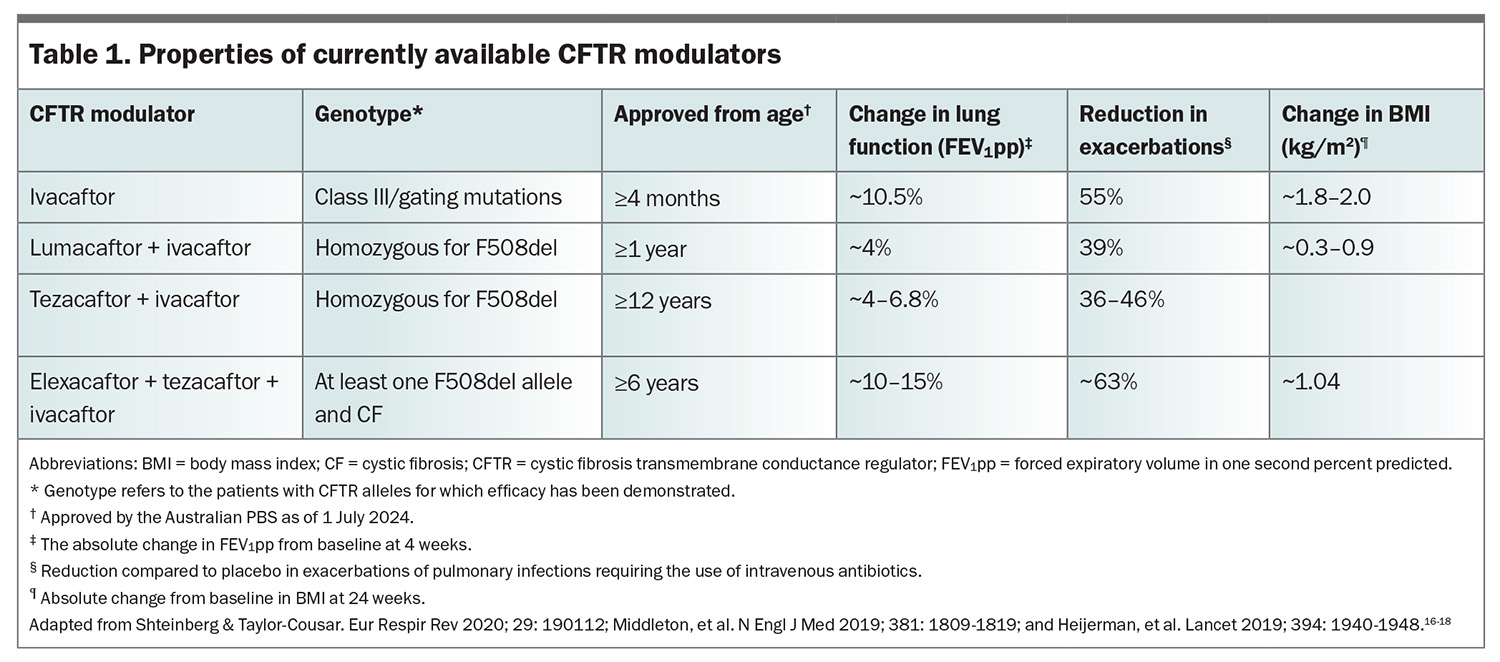
The advent of small-molecule CFTR modulators in the past decade promises to be transformational for people with CF. These modulators enhance CFTR function directly and for the first time treatments are available that specifically target the disease process. With their approval for use in ever younger populations who have not yet developed serious lung disease, they promise increased life expectancy.15 Information regarding these agents is summarised in Table 1.16-18
The discovery of CFTR modulators was the direct result of patient advocacy and fundraising that enabled the injection of $40 million into high-throughput drug screening. This screening identified target molecules that were predicted to improve CFTR function and such improvement was confirmed using human cell-based models.13
Ivacaftor
The first of these agents, ivacaftor, was found in trials to improve function of people with a class III/gating mutation found in about 5% of people with CF (Table 1).19, 20 These trials demonstrated improvements in lung function of approximately 10% from baseline (as measured by percentage predicted forced expiratory volume in one second [FEV1]), as well as improvements in weight and nutritional parameters and a reduction in the need for intravenous antibiotics for chest infections. These are important surrogate outcomes that have been shown to relate to mortality and healthcare utilisation.21 The efficacy of ivacaftor was sustained following its widespread introduction in the UK and USA since 2014, and this has allowed their national registry data to demonstrate reduced mortality, hospitalisation and the need for lung transplantation.22
Ivacaftor plus lumacaftor and ivacaftor plus tezacaftor
About 90% of people with CF have the class II mutation, with phenylalanine deletion at position 508 (F508del). The next generation of modulators were designed to specifically target people with this CFTR mutation. The first agents were a combination of ivacaftor and lumacaftor or ivacaftor and tezacaftor and targeted people homozygous for F508del.23 Their impact on lung function was not as great as that seen for ivacaftor nor their ability to reduce infection, but they did improve nutrition and their effect was sufficient to lead to their widespread implementation (Table 1).24,25
Elexacaftor plus tezacaftor plus ivacaftor
The development of the triple-combination modulator, elexacaftor, tezacaftor and ivacaftor (ETI), enhanced CFTR function by improving conformational protein folding as well as reducing protein destruction and expression at the cell surface. Development of ETI was a major next step, resulting in restoration of CFTR function in vitro in excess of 40% in cells homozygous for F508del, but was also substantially restored in cells from people with just one F508del mutation.26 This led to clinical trials of ETI that demonstrated improvements in the FEV1 percent predicted in the order of 10 to 15%, improved nutrition, improved quality of life and a reduction in pulmonary infections by more than 60% in those homozygous for F508del.17,18 These improvements were also seen in patients with one F508del mutation in combination with one of several mutations characterised by minimal function (MF) of the CFTR-encoded transporter, present in about 20% of people with CF (Table 1).17,18
The cogent case that had been made regarding the ability of these agents to correct the underlying disease process and their strong response clinically in short-term studies resulted in approval of ETI from 2019 onwards in the USA, Australia and Europe. Registry data have shown that even outside of trial settings ETI led to significant improvements in lung function, nutrition and quality of life, as well as to reductions in pulmonary infections, with these changes sustained for up to 30 months.27,28
Confidence in the clinical effectiveness of ETI has allowed the design of trials that demonstrate improvements in novel markers of lung function, such as the lung clearance index, a specialised test of pulmonary ventilation that can be performed in children as young as 4 years of age. This is critical in children in whom spirometry is essentially within normal limits and has proven to be a sensitive test to detect the presence of lung disease in children.29
In a randomised controlled trial, ETA showed a benefit compared with placebo in the lung clearance index consistent with an improvement in sweat chloride (a marker of CFTR function, measuring sweat gland ability to transport chloride ions) and quality of life in children 6 to 11 years of age.30 This enabled an open-label trial in children aged 2 to 5 years in which ETI was shown to be safe and tolerable in the short term and again led to improvements in the lung clearance index and sweat chloride test.31
These findings have been sufficient to allow approval of ETI in most jurisdictions in children as young as 2 years of age. It means that most people with CF will be on a modulator therapy in nearly all stages of life and will now be able to commence treatment before they have shown any changes in lung function. The hope is that this will substantially alter the risk of developing bronchiectasis and chronic infection with pathogens such as Pseudomonas, as well as preventing the accelerated loss of lung function later in life, factors that all result in increased mortality and the need for lung transplantation.
CFTR modulators (Table 1) are now available under the PBS section 100 scheme if prescribed by a physician expert in the care of people with CF and the patient is under the care of a CF specialist centre.
Interactions with CFTR modulators
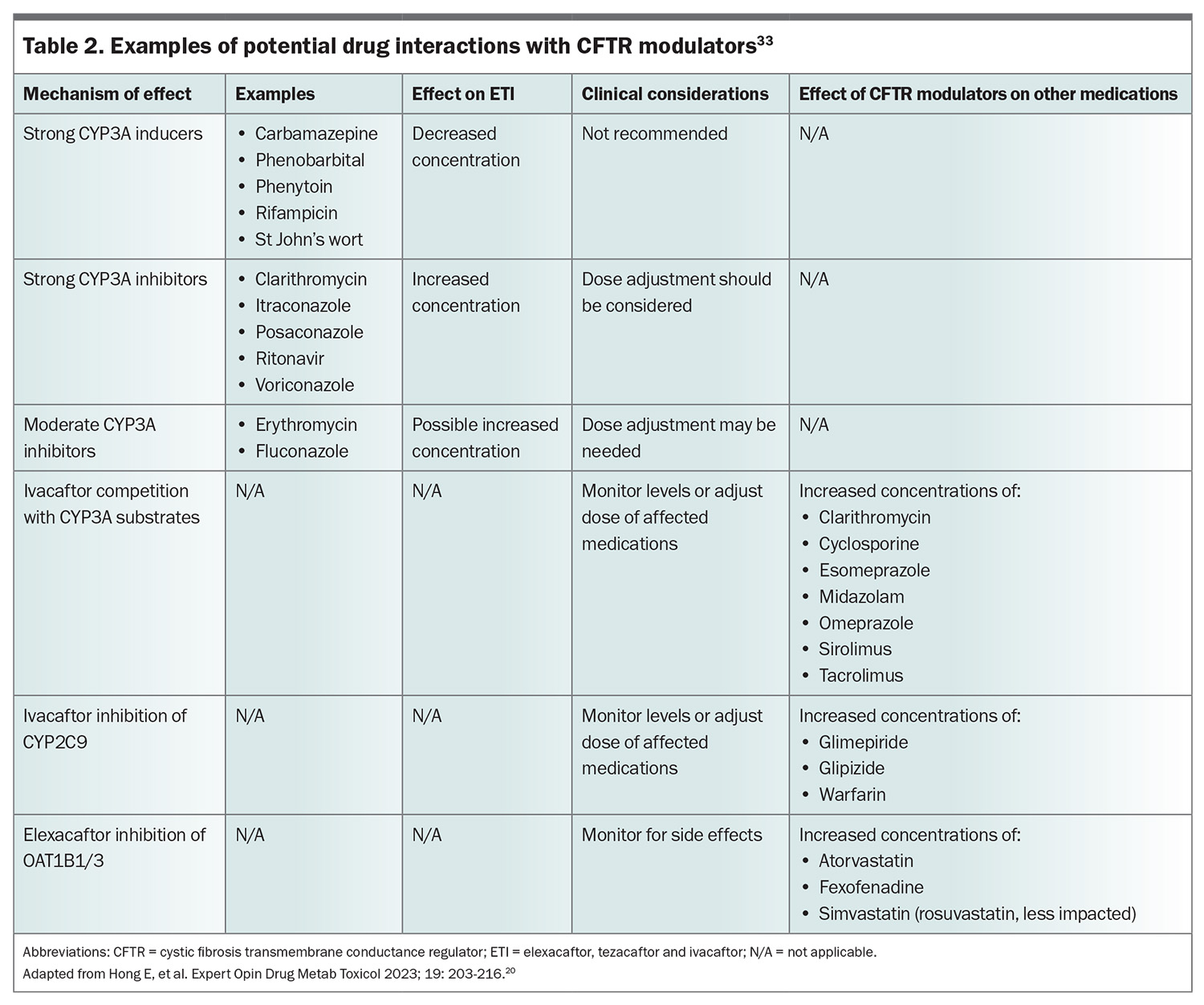
With the increasing numbers of people with CF, especially adult patients who are ageing, people with this disease will inevitably see their GPs for conditions other than CF. It is therefore important to consider medications that may interact with CFTR modulators. Elexacaftor, tezacaftor and ivacaftor are lipophilic and mostly metabolised by the liver through the cytochrome P450 CYP3A subfamily of enzymes.32 Therefore, CYP3A inducers will reduce levels of CFTR modulators, whereas inhibitors will potentially increase their levels. The CFTR modulators will also interact with other medications. The most important potential interactions that may require dose adjustment are summarised in Table 2.33
What about people in whom CFTR modulators will not work or who do not tolerate them?
CFTR modulators require CFTR protein to be made to correct or potentiate function. About 8% of the CF population in Australia are ineligible for modulators, as in most cases their mutations prevent the production of CFTR proteins.34 Some of these individuals will have rare mutations that were not included in the clinical trials but in whom modulators may be effective. The US Food and Drug Administration has taken the innovative step of accepting that in-vitro cell tests that demonstrate CFTR modulators are effective in those with rare mutations is a sufficient ground for approval, even without clinical data.35 However, this system is not without its limitations and the in-vitro model used has been criticised for not being an accurate reflection of clinical function. This was found to be the case when French investigators demonstrated clinical efficacy for ETI in an open-label report of people with a mutation that had been regarded as nonresponsive.36
An important component of this debate is the current cost of CFTR modulators. The Institute for Clinical and Economic Review (ICER) recommended the manufacturer of one of these modulators reduced the cost of its product as it found that although it was clinically effective, its very high cost exceeded its cost effectiveness and it was deemed ‘low long-term value for money at current prices’.37 In addition, people with CF from non-European backgrounds are less likely to have mutations that have been tested or will respond to CFTR modulators.38 This is highly relevant in Australia where access to CFTR modulators comes at public expense. Access should be provided to those who will benefit and to ensure that access is both just and equitable. It has been proposed that more accurate and personalised models of response to CFTR modulators are needed that more accurately predict both responders and nonresponders if there is clinical uncertainty.39 The inequity only worsens when the global perspective is considered, where access to standard CF care, let alone CFTR modulators, is largely limited to high-income countries, a moral and ethical gap that has largely gone unnoticed.38
For those people with mutations that lead to no CFTR protein production, CFTR modulators will never be effective. Several approaches that may address this are currently under investigation, including:
- agents that read through the abnormal gene code
- engineered formulations of CFTR RNA
- efforts to repair RNA
- the addition of CFTR DNA and gene editing technologies.40
What will CF care look like in the postmodulator world?
Despite these advances, considerable challenges remain for people with CF. Improved outcomes such as increased survival and the need for fewer transplants has meant the number of people entering adult CF centres will continue to increase steadily.34 Most adolescents and adults with CF currently have bronchiectasis and varying impairment of lung function and will still require monitoring and treatment of these complications. Reduced hospitalisations and stabilised lung function is shifting care to an outpatient setting and existing models of care will need to adapt.41
Nonpulmonary chronic conditions will assume greater prominence in an ageing CF population and will require more input from other health providers. More than one-third of adults with CF have diabetes requiring management and are at risk of future complications.34 People with CF are at increased risk of gastrointestinal malignancy, most often colorectal cancer, for which their risk is increased fivefold, requiring screening colonoscopies.42 Mental health concerns in CF have become increasingly apparent, with the prevalence of anxiety and depression estimated at 13 to 25%. This is not unique for people with a chronic disease, but with less acute disease it has emerged as a major unmet need.43 The current CF multidisciplinary team is not well equipped to deal with this and increasing help is needed from primary care and mental health providers to manage these problems.
Finally, a pleasing outcome of CFTR modulator therapy has, through better health and a direct effect leading to increased female fertility, resulted in a marked increase in pregnancy rates in women with CF.44 Overall, outcomes for both women with CF and their children are very good, but there is an increased risk of complications, such as gestational diabetes and low birth weight, leading to a need for increased monitoring across more maternity services.44 Inevitably, these increasing numbers of relatively well women will be seeking obstetric management from a broader range of maternity and primary care providers. As a result, people with CF will increasingly need support from a much broader range of adult health providers in both primary and secondary care.
Conclusion
Outcomes for people living with CF have dramatically improved. A child born with CF today could potentially expect to enjoy a normal life expectancy, albeit it one that requires ongoing medical support. Although this comes with the costs of highly specialised care, including the costs of CFTR modulators, we are seeing a generation of people with CF realising higher education goals and entering the full-time workforce, a generation of people who only 20 years ago were lost to society. This has been a story of remarkable success of cutting-edge science and discovery, but this was only made possible by the sacrifice and determination of the CF community.
Challenges remain for people with CF and those who care for them. Our highly specialised multidisciplinary clinics need to adapt to a rapidly changing clinical environment and we need to be able to convince health providers of the importance of doing this in a considered way so as not to lose the gains that have been made. Increasing numbers of people with CF will experience other health concerns that will require support from a broader range of health professionals who until now have had little exposure to people with CF. For those in whom CFTR modulators are not available or intolerance precludes their use, we need to strive for alternative treatments and consider innovative approaches to clinical trial design to find a solution for everyone with CF. RMT
COMPETING INTERESTS: Professor Wark has previously received honoraria from Vertex Pharmaceuticals.
References
1. Ahern S, Pourghaderi AR, Ruseckaiter R, et al, on behalf of the ACFDR. The Australia Cystic Fibrosis Data Registry Annual Report, 2023. Melbourne: School of Public Health and Preventive Medicine, Monash University, July 2024, Report No 25. Available online at: https://www.cysticfibrosis.org.au/wp-content/uploads/2024/07/ACFDR_2023_Annual-Report.pdf (accessed August 2024).
2. Scotet V, Gutierrez H, Farrell PM. Newborn screening for CF across the globe –where is it worthwhile? Int J Neonat Screening 2020; 6: 18.
3. Elborn JS. Cystic fibrosis. Lancet 2016; 388: 2519-2531.
4. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066-1073.
5. Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012; 487: 109-113.
6 Veit G, Avramescu RG, Chiang AN, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 2016; 27: 424-433.
7. The Clinical and Functional TRanslation (CFTR2). US CF Foundation, Johns Hopkins University, The Hospital for Sick Children; 2023. [Website]. Available at: https://cftr2.org/ (accessed August 2024).
8. O’Neal WK, Knowles MR. Cystic fibrosis disease modifiers: complex genetics defines the phenotypic diversity in a monogenic disease. Ann Rev Genomics Hum Genet 2018; 19: 201-222.
9. Kerem E, Corey M, Kerem BS, et al. The relation between genotype and phenotype in cystic fibrosis-analysis of the most common mutation (delta F508). N Engl J Med 1990; 323: 1517-1522.
10. Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168: 918-951.
11. Anderson DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Children 1938; 56: 344-399.
12. Allen L, Allen L, Carr SB, et al. Future therapies for cystic fibrosis. Nature Comm 2023; 14: 693.
13. Pallin M, Willis J. Innovative cystic fibrosis drug development: A perspective. Respirology 2022; 27: 1015-1017.
14. McBennett KA, Davis PB, Konstan MW. Increasing life expectancy in cystic fibrosis: advances and challenges. Pediatr Pulmonol 2022; 57 Suppl 1(Suppl 1): S5-S12.
15. Pettit RS, Fellner C. CFTR modulators for the treatment of cystic fibrosis. P t. 2014; 39: 500-511.
16. Shteinberg M, Taylor-Cousar JL. Impact of CFTR modulator use on outcomes in people with severe cystic fibrosis lung disease. Eur Respir Rev 2020; 29 : 190112.
17. Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-Tezacaftor-Ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019; 381: 1809-1819.
18. Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019; 394: 1940-1948.
19. Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013; 187: 1219-1225.
20. Ramsey BW, Davies J, McElvaney NG, Tullis E, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663-1672.
21. West NE, Flume PA. Unmet needs in cystic fibrosis: the next steps in improving outcomes. Expert Rev Respir Med 2018; 12: 585-593.
22. Bessonova L, Volkova N, Higgins M, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 2018; 73: 731-740.
23. Lopes-Pacheco M. CFTR modulators: shedding light on precision medicine for cystic fibrosis. Front Pharmacol 2016; 7. Available online at: https://doi.org/10.3389/fphar.2016.00275 (accessed August 2024).
24. Rehman A, Baloch NU, Janahi IA. Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373: 1783.
25. Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017; 377: 2013-2023.
26. Ridley K, Condren M. Elexacaftor-tezacaftor-ivacaftor: The first triple-combination cystic fibrosis transmembrane conductance regulator modulating therapy. J Pediatr Pharmacol Ther 2020; 25: 192-197.
27. Nichols DP, Paynter AC, Heltshe SL, et al. Clinical effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: a clinical trial. Am J Respir Crit Care Med 2022; 205: 529-539.
28. Sutharsan S, Dillenhoefer S, Welsner M, et al. Impact of elexacaftor/tezacaftor/ivacaftor on lung function, nutritional status, pulmonary exacerbation frequency and sweat chloride in people with cystic fibrosis: real-world evidence from the German CF Registry. Lancet Reg Health Eur 2023; 32: 100690.
29. Foong RE, Harper AJ, Skoric B, et al. The clinical utility of lung clearance index in early cystic fibrosis lung disease is not impacted by the number of multiple-breath washout trials. ERJ Open Res 2018; 4: 00094-2017.
30. Mall MA, Brugha R, Gartner S, et al. Efficacy and Safety of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis heterozygous for F508del and a minimal function mutation: a phase 3b, randomized, placebo-controlled study. Am J Respir Crit Care Med 2022; 206: 1361-1369.
31. Goralski JL, Hoppe JE, Mall MA, et al. Phase 3 open-label clinical trial of elexacaftor/tezacaftor/ivacaftor in children aged 2-5 years with cystic fibrosis and at least one F508del Allele. Am J Respir Crit Care Med 2023; 208: 59-67.
32. van der Meer R, Wilms EB, Heijerman HGM. CFTR modulators: does one dose fit all? J Pers Med 2021; 11: 458.
33. Hong E, Shi A, Beringer P. Drug-drug interactions involving CFTR modulators: a review of the evidence and clinical implications. Expert Opin Drug Metab Toxicol 2023; 19: 203-216.
34. Ahern S, Pourghaderi AR, Caruso M, et al, on behalf of the ACFDR. The Australia Cystic Fibrosis Data Registry Annual Report Registry Annual Report, 2022. Melbourne: Department of Epidemiology and Preventive Medicine, Monash University, December 2023, Report No 2.
35. Van Goor F, Yu H, Burton B, Hoffman BJ. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros 2014; 13: 29-36.
36. Sadras I, Kerem E, Livnat G, et al. Clinical and functional efficacy of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis carrying the N1303K mutation. J Cystic Fibros 2023; 22: 1062-1069.
37. Tice JA, Kuntz KM, Wherry K, Seidner M, Rind DM, Pearson SD. The effectiveness and value of novel treatments for cystic fibrosis. J Manag Care Spec Pharm 2021; 27: 276-280.
38. McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol 2021; 56: 1496-503.
39. Kaiko GE, Wark PAB. Developments in cystic fibrosis personalised epithelial assays: science and patient perspectives. J Cyst Fibros 2018; 17: 289-291.
40. Fajac I, Sermet I. Therapeutic approaches for patients with cystic fibrosis not eligible for current CFTR modulators. Cells 2021; 10: 2793.
41. Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020; 8: 65-124.
42. Hadjiliadis D, Khoruts A, Zauber AG, Hempstead SE, Maisonneuve P, Lowenfels AB. Cystic Fibrosis Colorectal Cancer Screening Consensus Recommendations. Gastroenterol 2018; 154: 736-745.e14.
43. Guta MT, Tekalign T, Awoke N, Fite RO, Dendir G, Lenjebo TL. Global burden of anxiety and depression among cystic fibrosis patient: systematic review and meta-analysis. Int J Chronic Dis 2021; 2021: 6708865.
44. Gur M, Pollak M, Bar-Yoseph R, Bentur L. Pregnancy in cystic fibrosis-past, present, and future. J Clin Med 2023; 12: 1468.