Community-acquired pneumonia: GP care after the pandemic
Community-acquired pneumonia is common and most patients can be managed in the community. Management decisions for GPs include: Does the patient need to go to hospital? Should I organise a chest x-ray, viral PCR testing or sputum culture? What antibiotics should I prescribe? How should the patient be followed up?
- Amoxicillin remains the empirical antibiotic of choice to treat patients with community-acquired pneumonia.
- Pulse oximetry is noninvasive and readily available and should be used in initial patient assessment to help determine disease severity.
- An episode of pneumonia presents an opportunity to modify the patient’s risk factors, including smoking, high alcohol intake and poor dental hygiene.
- If a chest x-ray shows abnormalities, it should be repeated at six weeks to confirm resolution.
Pneumonia is a common condition seen in the community and is associated with significant morbidity and mortality. In 2020, lower respiratory tract infection caused 100,388 hospitalisations (a rate of 390 per 100,000 people) and 2467 deaths in Australia.1 Presentations with community-acquired pneumonia have likely increased with the COVID-19 pandemic, which has also shown the value of rapid nucleic acid amplification techniques for diagnosis and management. Appropriate triage and early treatment are important to protect our patients.
This article explores what primary care providers need to know to assess and manage patients with community-acquired pneumonia, incorporating local and international guidelines and our clinical experience. The guidance applies to nonimmunocompromised adults with minimal comorbidities and no significant underlying lung disease. The main decisions for the primary care provider at the first presentation of a patient with suspected pneumonia are: Should I give antibiotics? Should I organise a chest x-ray, viral PCR test or sputum culture? Does the patient need to go to hospital? How should they be followed up?
What is pneumonia?
Pneumonia refers to acute inflammation of the lung parenchyma caused by an infectious micro-organism, resulting in tissue damage and physiological derangement. It is defined by compatible clinical symptoms and radiological confirmation of pulmonary consolidation. In primary care, it is difficult to differentiate pneumonia at first presentation from other causes of lower respiratory tract infection, such as bronchitis, bronchiolitis and exacerbation of airways disease (asthma, chronic obstructive pulmonary disease and bronchiectasis).
Pneumonia is commonly caused by viruses or bacteria, and less often by fungal organisms. In cases of viral infection, the upper respiratory tract symptoms of rhinitis and laryngitis and systemic symptoms may accompany the presentation. Viral pneumonia can cause dysfunction in the usual protective mechanisms in the lung and lead to superimposed bacterial infection.
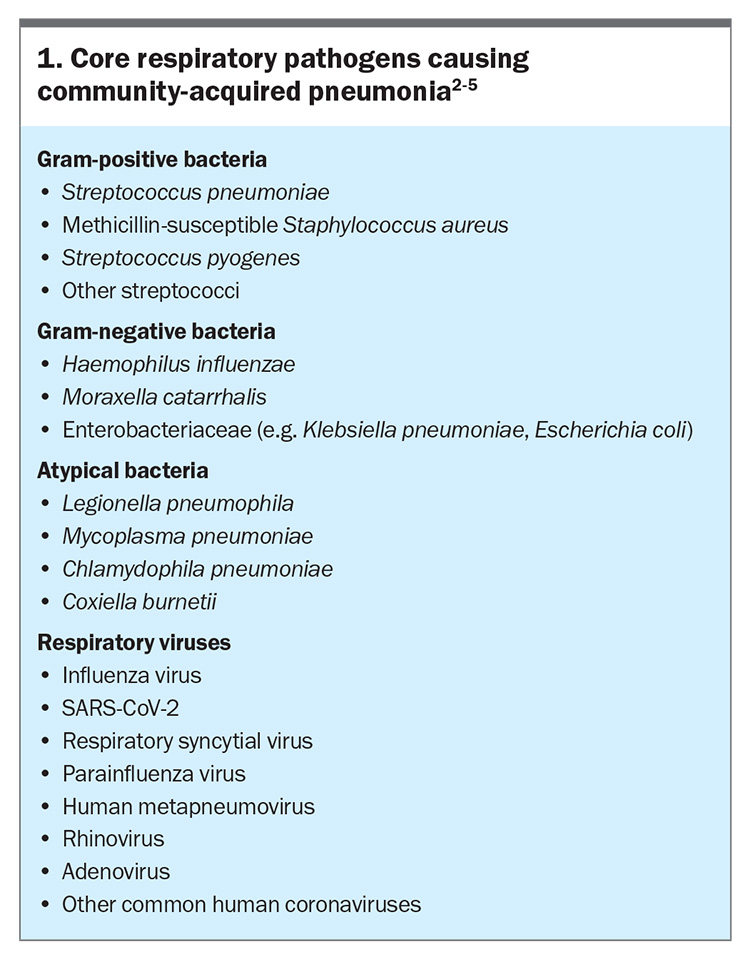
Micro-organisms can access the lower respiratory tract and cause infection by two main routes. The main route for typical bacteria, including Streptococcus pneumoniae, is microaspiration of nasopharyngeal organisms, particularly during sleep. Viruses and atypical organisms (Legionella spp. and Mycoplasma spp.) reach the lower respiratory tract via inhalation in the setting of environmental exposure. These micro-organisms overcome local innate defences, including mucus trapping, the mucociliary ‘escalator’ and local immune cells, to cause tissue injury and an exacerbated inflammatory response. Core pathogens that cause community-acquired pneumonia are listed in Box 1.2-5
Modifiable risk factors for pneumonia include smoking, alcohol misuse, being underweight, regular contact with children and poor dental hygiene.6 Nonmodifiable risk factors include increasing age and comorbid conditions; those associated with the highest risk include previous pneumonia, chronic respiratory disease and chronic cardiovascular disease.6
Pneumonia presents with a combination of shortness of breath, cough, sputum production, fever, malaise, anorexia, pleuritic chest pain and confusion. Acute bronchitis and exacerbation of airways disease can have similar symptoms. Accompanying symptoms of wheeze, chest tightness or change in the usual sputum colour or amount may point to an exacerbation of underlying airways disease. It is difficult to determine whether this lower respiratory syndrome reflects pneumonia or to identify the causative organism without imaging or microbiological investigation. Although certain features may lead physicians to consider less common causative organisms, the terms ‘typical’ and ‘atypical’ pneumonia are discouraged because clinical examination and radiology are not sensitive or specific enough to be able to determine the cause.7
Pulse oximetry is readily available and can assist in the rapid assessment of pneumonia severity. Hypoxaemia, tachypnoea and new or worsening confusion indicate more severe disease. Signs of haemodynamic stability should be sought through blood pressure and heart rate measurement. Other examination findings in pneumonia include local signs of lung parenchymal involvement with crepitations, rales and dullness to percussion.
Who should be sent to hospital?
Clinical assessment of the patient at the bedside is essential in determining whether the patient can be safely treated in the community or needs hospitalisation, and their risk of deterioration. Several scoring systems have been designed to identify patients at higher risk of mortality from pneumonia, and these can be used to supplement clinical assessment and decision making.
The Pneumonia Severity Index (PSI) is supported by the most evidence and is the preferred score to determine the location of care.8 Developed in the US, the PSI assesses 20 variables to stratify patients into risk categories based on 30-day mortality. However, the CRB65 score is easier to apply in the community; it includes five risk factors, each of which scores one point to a maximum score of 5: new-onset confusion, respiratory rate 30 breaths per minute or greater, low systolic (less than 90 mmHg) or diastolic (60 mmHg or less) blood pressure, and age over 65 years.9 The CURB65 score includes an additional risk factor, urea level greater than 7 mmol/L. A meta-analysis showed no significant differences between the PSI, CRB65 and CURB65 scores in predicting mortality.10
British Thoracic Society guidelines recommend assessment of the CRB65 score; patients with a CRB65 score of 0 can be managed in the community, those with a score of 2 should be considered for hospital assessment and those with a score of 3 or more need urgent hospital assessment.11 For patients with a score of 1, clinical judgement and discussion with the patient are recommended. If the patient’s CRB65 score is low and they have no red flags (Box 2),12 we recommend outpatient management with early follow up.
Who needs a chest x-ray?
Although a chest x-ray showing pulmonary consolidation is required to make the diagnosis of pneumonia, most patients with suspected pneumonia managed in the community do not need chest imaging, as they are generally healthy with mild symptoms that resolve with empirical treatment. Chest radiography should be performed in all patients presenting to hospital with symptoms consistent with pneumonia. In the outpatient setting, a chest x-ray can be considered when the diagnosis is in doubt, when the patient is not improving as expected and there is suspicion of multilobar pneumonia, an effusion or other complications, and when the patient is at risk of underlying lung cancer.11 It is also reasonable to perform a chest x-ray in patients with a CRB65 score greater than 0, which can help with decisions about location of care.
When should I do a viral PCR test and sputum culture?
Guidelines from before the COVID-19 pandemic recommended against PCR testing to identify the cause for patients with pneumonia in the absence of an influenza outbreak.13 The advice was similar for urinary antigen testing in the absence of a legionella outbreak. With the experience of SARS-CoV-2, rapid nucleic acid amplification technologies have advanced, as well as vaccination and treatment of viral illnesses. Viruses are a common cause of pneumonia, and the availability or medications that can change the outcome for two of these (influenza virus and SARS-CoV-2) has made routine PCR testing more appealing. Multiplex nucleic acid amplification panels can also target atypical bacteria.4 Further research is needed to determine the impact of these tests on outcomes or antibiotic stewardship. However, in a patient with suspected COVID-19 or seasonal influenza or whose condition is not improving with empirical therapy, a multiplex nucleic acid amplification panel for respiratory viruses should be performed.
Sputum is not routinely cultured from ambulatory patients unless there is concern about antibiotic-resistant bacteria (Pseudomonas spp. and methicillin-resistant Staphylococcus aureus) or atypical organisms (Legionella spp. and Chlamydia spp.) are considered more likely based on clinical features. Sputum should be cultured when patients do not respond to empirical therapy.
Which antibiotic should I use?
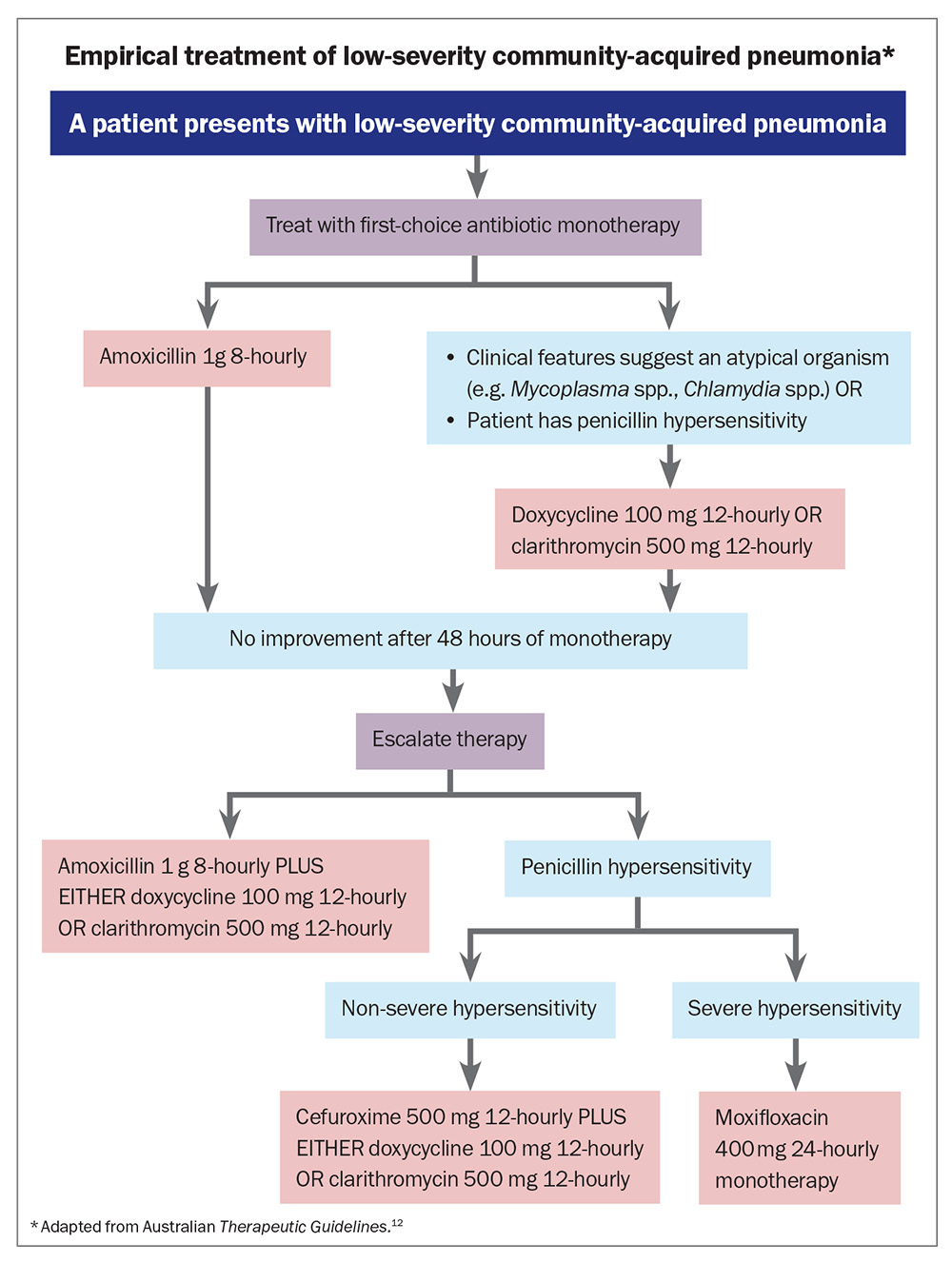
The choice of antibiotic treatment should attempt to cover the most likely organisms effectively. Australian Therapeutic Guidelines currently recommend monotherapy for patients with low-severity community-acquired pneumonia (Flowchart).12 This is consistent with the
American Thoracic Society and Infectious Diseases Society of America (ATS/ISDA) guidelines.14 The drug of choice is amoxicillin 1 g orally 8-hourly because of increasing rates of S. pneumoniae resistance to macrolides and tetracyclines. If atypical pathogens are considered more likely based on clinical features, or the patient has hypersensitivity to penicillin, doxycyline 100 mg orally 12-hourly or clarithromycin 500 mg orally 12-hourly is recommended.
If the patient’s condition has not improved after 48 hours, escalate to combined therapy with amoxicillin plus doxycycline or clarithromycin. For patients who are unable to be reviewed at 48 hours after treatment initiation, it is reasonable to start with combination therapy, given the prevalence of atypical organisms.
The duration of therapy should be five to seven days in patients with no complications. If there is no improvement at five days, the patient should be investigated for complications, including empyema or lung abscess, initially with a chest x-ray, possibly followed by a CT scan if there are concerning features. Causes of non-resolving pneumonia include underlying bronchogenic carcinoma with airway obstruction, empyema and infection with less common organisms that are not susceptible to guideline-directed empirical therapy (e.g. tuberculosis), or inflammatory pneumonia (e.g. vasculitis, organising pneumonia).
Empirical macrolide antibiotic treatment for community-acquired pneumonia is controversial. Amoxicillin will treat S. pneumoniae, which is the most likely cause. Macrolides are reserved for cases where atypical organisms are suspected. However, pneumonia caused by atypical organisms is also common enough that it can be argued to use a macrolide upfront in addition to amoxicillin. Despite this, both the Australian Therapeutic Guidelines and ATS/ISDA guidelines suggest amoxicillin monotherapy as the first-line treatment for community-acquired pneumonia in healthy outpatient adults without comorbidities or risk factors for antibiotic resistant pathogens.12,14
Early clinical review
When possible, patients with suspected pneumonia should be reassessed after 48 hours to confirm improvement and monitor for any deterioration.11 If symptoms have worsened or there is clinical concern, we recommend performing a chest x-ray and sputum culture at this time. If the patient has any red flags or a CRB65 score of 2 or more, consider referring them to hospital. If there is no improvement despite amoxicillin monotherapy then a tetracycline or macrolide should be added to therapy.
Risk factor modification
An episode of pneumonia presents an opportunity to discuss risk factors, particularly smoking. Smoking increases the risk of community-acquired pneumonia by a factor of 1.34 for ex-smokers and 1.37 for current smokers.15 The risk of developing pneumonia is lower in ex-smokers who have quit for more than four years compared with those who have quit within the previous year (odds ratio 0.39).15 The increase in risk of pneumonia with vaping is not known, but it is likely to disrupt airway defence mechanisms against micro-organisms similarly to smoking. High alcohol intake and poor dental hygiene are other modifiable risk factors that should be addressed in follow up.
An episode of pneumonia is associated with an increased risk of cardiovascular disease, particularly in older patients.16,17 It would be prudent to assess cardiovascular risk, including lipid status and blood pressure, and the need for therapies such as aspirin or cholesterol-lowering therapy.
What follow up is needed?
Uncomplicated, low-severity pneumonia does not require routine follow up if all symptoms have clinically resolved and the patient is generally healthy with no obvious modifiable risk factors. In patients who were sick enough to warrant a chest x-ray, a follow-up chest x-ray should be performed at six weeks to ensure resolution and evaluate for malignancy as an underlying cause of the infection. Patients with recurrent infections or persistent radiological abnormalities warrant referral to a respiratory physician.
Vaccination
Vaccines are available against S. pneumoniae, influenza virus, SARS-CoV-2 and respiratory syncytial virus (RSV). A new recombinant RSV vaccine was recently approved by the TGA for patients older than 60 years. This vaccine has 94.1% efficacy against severe RSV-related lower respiratory tract disease in community-dwelling patients aged over 60 years.18 Current Australian recommendations on vaccination to help prevent pneumonia in adults from the Australian Technical Advisory Group on Immunisation (ATAGI) are summarised in the Table.19,20
When should I refer a patient to a respiratory physician?
Most patients with lower respiratory tract infection can be managed in primary care. Triggers for referral of patients to specialist care include:
- non-resolving pneumonia
- recurrent pneumonia
- lung abscess
- suspected bronchogenic carcinoma
- frequent exacerbations of airways disease (two or more moderate exacerbations per year).
Conclusion
Pneumonia remains a common cause of disease in community dwelling adults, both those with chronic disease and those who are otherwise well. Most patients with pneumonia can be treated in the community, but it is important to recognise indications for hospitalisation and for specialist follow up. GPs play a crucial role in reducing pneumonia incidence through vaccination of their patients and risk factor modification. RMT
COMPETING INTERESTS: Professor Morgan has received speaker fees from Insmed, AstraZeneca, Novartis, GSK and Zambon and research grants
from AstraZeneca, Boehringer Ingelheim and Novartis. Dr Holmes: None.
References
1. Australian Institute of Health and Welfare (AIHW). Infectious and communicable diseases. Canberra: AIHW; 2022. Available online at: https://www.aihw.gov.au/reports/australias-health/infectious-and-communicable-diseases (accessed April 2024).
2. File TM Jr, Ramirez JA. Community-acquired pneumonia. N Engl J Med 2023; 389: 632-641.
3. Ramirez JA, Musher DM, Evans SE, et al. Treatment of community-acquired pneumonia in immunocompromised adults. Chest 2020; 158: 1896–1911.
4. Gadsby NJ, Musher DM. The microbial etiology of community-acquired pneumonia in adults: from classical bacteriology to host transcriptional signatures. Clin Microbiol Rev 2022; 35: e0001522.
5. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373: 415-427.
6. Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013; 68: 1057-1065.
7. Bochud PY, Moser F, Erard P, et al. Community-acquired pneumonia: a prospective outpatient study. Medicine (Baltimore) 2001; 80: 75-87.
8. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243-250.
9. Lim WS. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377-382.
10. Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010; 65: 878-883.
11. Lim WS, Baudouin SV, George RC, et al; Pneumonia Guidelines Committee of the BTS Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64 Suppl 3: iii1-iii55.
12. Therapeutic guidelines: antibiotic. Version 16. Melbourne: Therapeutic Guidelines Ltd; 2024. Available online at: https://www.tg.org.au (accessed April 2024).
13. Evans SE, Jennerich AL, Azar MM, et al. Nucleic acid–based testing for noninfluenza viral pathogens in adults with suspected community-acquired pneumonia. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2021; 203: 1070-1087.
14. Olson G, Davis AM. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA 2020; 323: 885-886.
15. Almirall J, Bolibar I, Serra-Prat M, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J 2008; 31: 1274-1284.
16. Eurich D, Marrie T, Minhas-Sandhu K, et al. Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up. BMJ 2017; 356: j413.
17. Corrales-Medina V, Alvarez K, Weissfeld L, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313: 264-274.
18. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion f protein vaccine in older adults. N Engl J Med 2023; 388: 595-608.
19. Australian Technical Advisory Group on Immunisation (ATAGI). Australian immunisation handbook. Canberra: Australian Government Department of Health and Aged Care; 2022. Available online at: https://immunisationhandbook.health.gov.au (accessed April 2024).
20. Australian Technical Advisory Group on Immunisation (ATAGI). ATAGI statement on the clinical use of Arexvy (RSV PRE-F3) vaccine for RSV. Version 1.0. Canberra: Australian Government Department of Health and Aged Care; 2024. Available online at: https://www.health.gov.au/resources/publications/atagi-statement-on-the-clinical-use-of-arexvy-rsv-pre-f3-vaccine-for-rsv?language=en (accessed April 2024).