Tuberculosis: don’t miss the signs
Although Australia has a low incidence of tuberculosis, the infection should be considered in patients with risk factors or symptoms. GPs play an integral role in recognising tuberculosis and its various presentations, screening for latent tuberculosis infection and identifying patients with active tuberculosis disease for referral and treatment.
- Tuberculosis infection is present in the community in Australia, with a notification rate of 6.9 cases per 100,000 in 2021.
- Most people infected with Mycobacterium tuberculosis will not develop active tuberculosis disease; a proportion clear the infection and some develop latent tuberculosis infection (LTBI), with a 5 to 10% lifetime risk of progressing to active disease.
- Screening of people with risk factors for LTBI by tuberculin skin testing or interferon-gamma release assay is an important component of tuberculosis control; management of LTBI involves chemoprophylaxis or clinical and radiological surveillance.
- Active tuberculosis disease most commonly presents as a pulmonary infection or lymphadenitis but can involve any organ system except the hair and nails.
- Investigation for active tuberculosis disease includes physical examination, chest x-ray, microscopy and culture of sputum or other tissue for acid-fast bacilli, and molecular testing.
- Treatment of active disease requires multidrug therapy with a patient-centred multidisciplinary approach.
Tuberculosis is a significant issue worldwide. An estimated 1.7 billion people, or about 22% of the world population, are infected with Mycobacterium tuberculosis, the airborne pathogen that causes tuberculosis disease.1 Most of those infected will not develop active tuberculosis disease; a proportion clear the infection and some develop latent infection, with the remainder progressing to active disease. Tuberculosis is predominantly an issue of developing nations, with most of the 10.6 million notifications of active tuberculosis and 1.6 million deaths in 2021 occurring in lower income countries.1
Tuberculosis infection is present in the community in Australia, with a notification rate of 6.9 cases per 100,000 in 2021 according to WHO data.1 Further, individuals may develop active tuberculosis disease many years after contracting the infection overseas or domestically in the past when prevalence rates were much higher.
GPs have an important role in recognising tuberculosis and its various presentations, screening for latent infection and identifying people with active disease and referring them to a public health unit.
Spectrum of tuberculosis infection
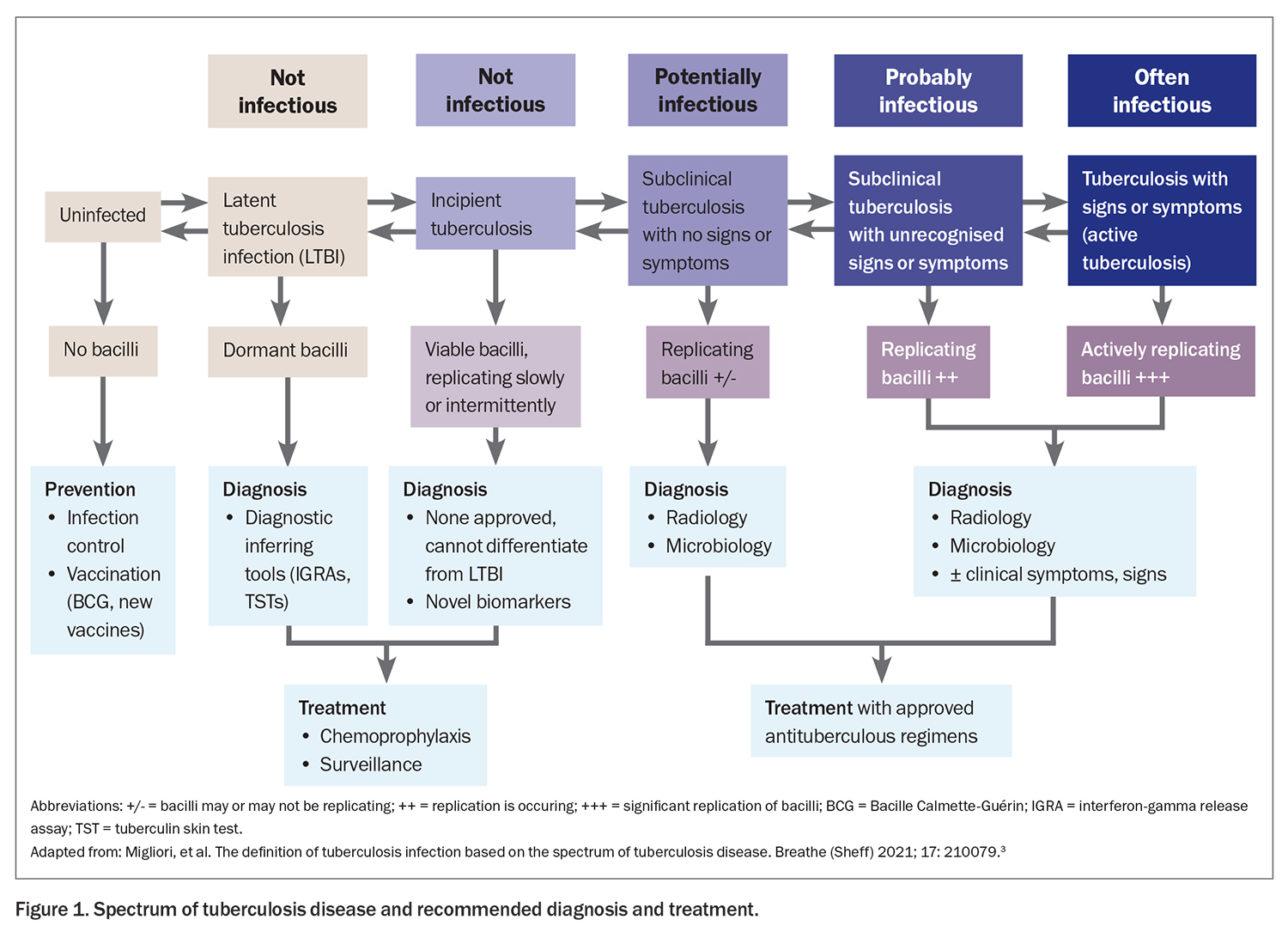
Historically, tuberculosis infection was thought of in binary terms: latent or active. Nowadays, we have realised this oversimplifies its pathogenesis. Tuberculosis infection is better conceived as a continuum, with latent infection at one end and active disease at the other (Figure 1).2-7 Between these two disease states are differing stages of bacillary viability and replication.
- Latent tuberculosis infection (LTBI) is a state of persistent immune response to stimulation by M. tuberculosis antigens with no evidence of clinically manifest active tuberculosis (in terms of symptoms, x-ray changes or microbiological evidence). The bacilli are dormant and not replicating, and individuals are not considered infectious to others.
- Incipient tuberculosis infection occurs when there are viable M. tuberculosis bacteria but they alternate between periods of dormancy, as in latent infection, and periods of slow metabolic activity and replication, with no clinical symptoms, radiographic abnormalities or microbiological evidence consistent with active tuberculosis disease. Currently, no validated tools or biomarkers are available to differentiate incipient tuberculosis infection from latent infection.
- Subclinical tuberculosis disease refers to a state where abnormalities are present to suggest viable bacteria (chest x-ray changes, microbiological evidence) but the individual has no clinical symptoms. M. tuberculosis may be cultured from specimens, and some individuals may be infectious despite the absence of symptoms. Subclinical tuberculosis can be detected through chest x-ray screening as well as sputum screening in close contacts of patients with tuberculosis.
- Active tuberculosis disease is defined as the presence of symptoms and radiological or microbiological evidence of M. tuberculosis infection. People with untreated tuberculosis of the respiratory tract are the source of transmission in essentially all new cases of tuberculosis infection and can infect up to one-third of their household contacts.
Factors that lead to transitioning between these disease states include host innate and acquired immunity as well as the metabolic activity of the M. tuberculosis organism.2,3
Screening for latent tuberculosis infection
Screening for LTBI is an important component of tuberculosis control. About 5 to 10% of people with LTBI develop active tuberculosis disease over their lifetime, with the risk for developing active disease dependent on bacterial, host and environmental factors.4,8-10 Further, in countries where the overall prevalence of tuberculosis is low, such as Australia and New Zealand, reactivation of LTBI accounts for most new cases of tuberculosis.11 Detection of LTBI and treatment of affected populations is therefore an important component of the WHO End TB Strategy.12
Who should be screened for LTBI?
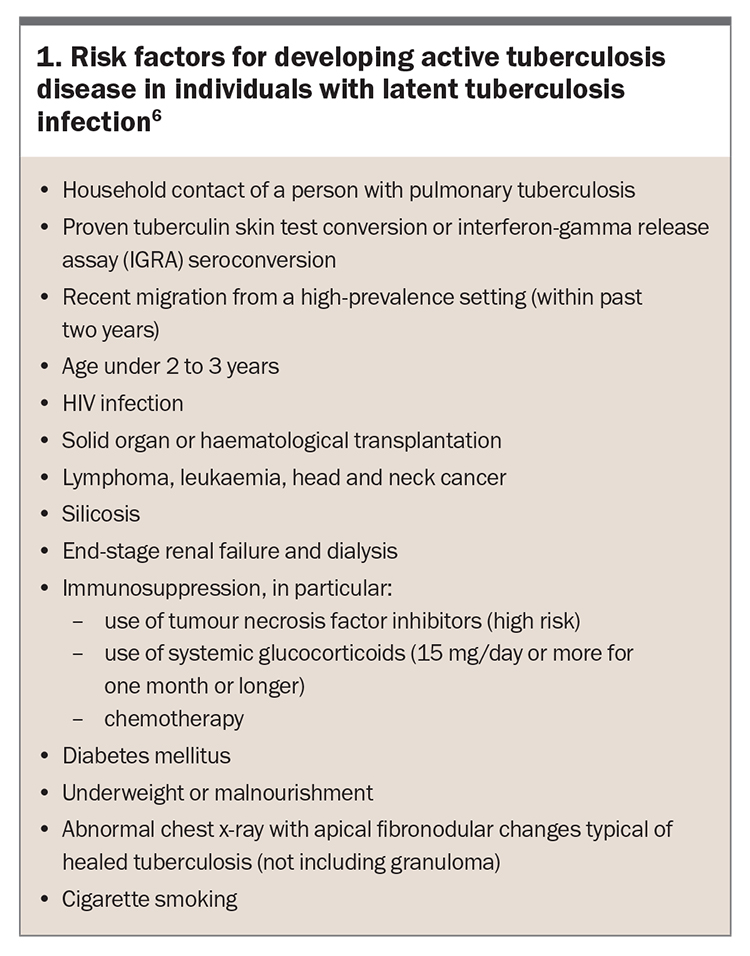
Risk factors for developing active tuberculosis disease are shown in Box 1. Patients at highest risk will most benefit from LTBI screening and management. Immunological status is one of the most important predictors of risk of progression from LTBI to active tuberculosis disease.4,13 Factors affecting cellular immunity such as HIV infection, solid organ or haematological transplantation, use of tumour necrosis factor inhibitors, prolonged corticosteroid use, diabetes mellitus and end-stage renal disease are all known to increase the risk of progression to active tuberculosis disease (Box 1).6 Smoking is also considered a risk factor for tuberculosis.14,15
Most guidelines recommend screening people born in high-burden tuberculosis countries who possess one or more risk factors, in whom preventive treatment is most likely to be of benefit.12,14 In addition, screening of people with occupational risk factors such as healthcare workers is now common.
How should you screen for LTBI?
There is no gold-standard test for the diagnosis of LTBI. The WHO recommends either tuberculin skin testing or interferon-gamma release assay (IGRA) as part of a screening strategy for LTBI.12,16 In general, the IGRA is more commonly used in Australia and New Zealand because of its practicality (only one visit is needed) and high specificity for tuberculosis infection.
The tuberculin skin test (using the Mantoux technique) involves the intradermal injection of tuberculin and measurement of a delayed-type hypersensitivity response within a defined time interval.10,16 The tuberculin purified protein derivative used for the injection shares some homologous antigens with the Mycobacterium bovis strain used in the Bacille Calmette-Guérin (BCG) vaccination and some other nontuberculous mycobacteria, which can lead to false-positive results. These are thought to be less common in the decades after vaccination.16,17
The IGRA blood test (e.g. QuantiFERON-TB Gold In-Tube and T-Spot.TB test) uses antigens that are more specific to the M. tuberculosis complex, the group of Mycobacterium species that can cause tuberculosis in humans or other animals, such as Mycobacterium bovis and the BCG strain. However, these antigens are shared with some nontuberculous mycobacteria, including Mycobacterium marinum, M. szulgai, M. flavescens and M. kansasii.10 On balance, the IGRA is more specific than tuberculin skin testing.18-21 However, both are commonly used in clinical practice.12,16
As both tuberculin skin testing and IGRA testing rely on cell-mediated immunity, they require a competent immune response to reliably identify people with tuberculosis infection. For example, an indeterminate IGRA result can occur when there is a high background interferon-gamma response to the negative control (e.g. in acute illness or for unknown reasons) or a low interferon-gamma response to the positive (mitogen) control (e.g. in immunosuppression). In these situations, the test might need to be repeated. If the result is persistently indeterminate, an assessment of the probability of latent infection is required, and referral to a specialist tuberculosis centre may be appropriate.
A key point to note is that both tests are likely to give positive results indefinitely, even after appropriate treatment. Also, neither of the screening tests for LTBI can distinguish between latent versus active tuberculosis, and screening tests may even have negative results in individuals with active disease.
Assessment of patients identified by screening
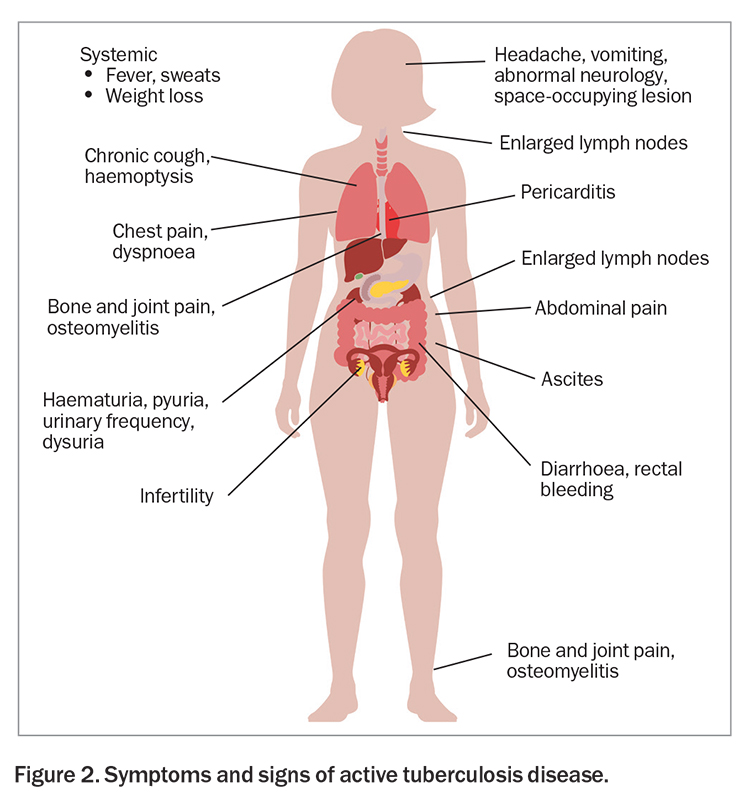
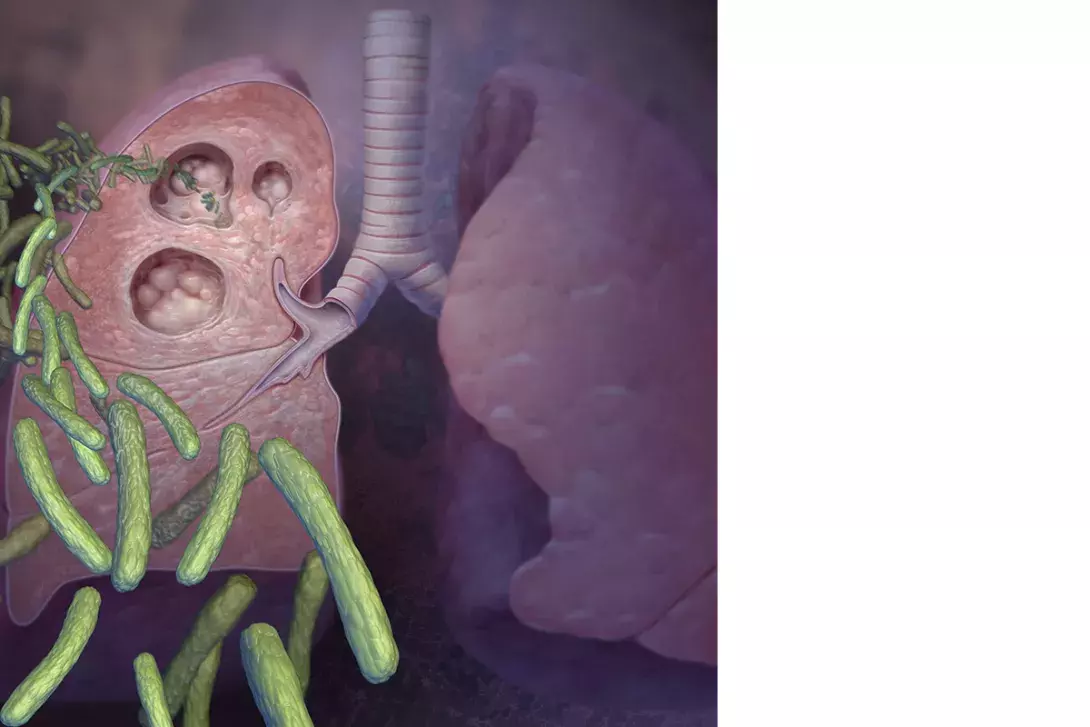
In patients with a positive result on a tuberculosis screening test, it is important to exclude active disease.12 GPs should ask about possible symptoms of tuberculosis, particularly chronic cough (longer than one month), haemoptysis, night sweats, unintentional weight loss and persistently enlarged lymph nodes. Tuberculosis can affect any organ in the body other than the hair and nails but most commonly presents as pulmonary disease, with lymphadenitis the most common extrapulmonary manifestation (Figure 2).
Any unexplained symptoms must be taken seriously in a person with epidemiological risk and a positive result on tuberculosis screening. A chest x-ray should be performed to exclude subclinical (asymptomatic) disease. If the patient has a cough or chest x-ray changes, three consecutive sputum samples should be assessed for acid-fast bacilli (AFB) by microscopy (smear) and culture (see below). Referral to a specialist tuberculosis centre is often a requirement of occupational screening for medical clearance.
If there are no signs, symptoms or radiological changes to suggest active tuberculosis disease then the diagnosis is LTBI.
Management of LTBI
There are two approaches to the management of LTBI:
- chemoprophylaxis with a course of preventive antibiotics (e.g. rifampicin, isoniazid, a combination of these or weekly rifapentine plus isoniazid)
- a two-year period of clinical and radiological surveillance, involving six-monthly assessment of symptoms, physical examination (particularly chest auscultation and examination of lymph nodes) and chest x-ray.
Given that only 5 to 10% of people with LTBI will develop active tuberculosis disease over their lifetime, the choice whether to offer chemoprophylaxis with its potential for adverse effects needs to be informed by an assessment of the individual’s risk of developing active disease.6,10,12,22 The risk of progression from latent infection to active tuberculosis disease is highest close to the time of acquisition of the infection, with most of the risk occurring within the first two years after initial exposure.23 The benefit of chemoprophylaxis is therefore highest in recently infected individuals, recent emigrants from high to low tuberculosis-burden countries and those with other risk factors for progression (Box 1).6,12,24-27
The Online TST/IGRA Interpreter is a useful tool to estimate an individual’s risk of developing active tuberculosis (www.tstin3d.com).28 This tool takes into account risk factors such as country of birth, age at migration to a low tuberculosis-burden country and risk factors for reactivation. A decision to start chemoprophylaxis must also take into account the potential for adverse effects, which is affected by age and comorbidities, and the potential for drug interactions.
Case studies that illustrate decision-making about chemoprophylaxis in patients with LTBI are described in Box 2 and Box 3.
How effective is chemoprophylaxis in preventing active tuberculosis?
Trials of chemoprophylaxis in people with LTBI have shown a 60 to 90% reduction in the incidence of active tuberculosis compared with placebo.4,6,29,30 Efficacy is mostly influenced by adherence rate. The treatments are safe and well tolerated overall. Isoniazid-induced hepatotoxicity is the main concern, with rates varying from 0.1 to 1% during treatment for LTBI.31
What chemoprophylaxis options are available?
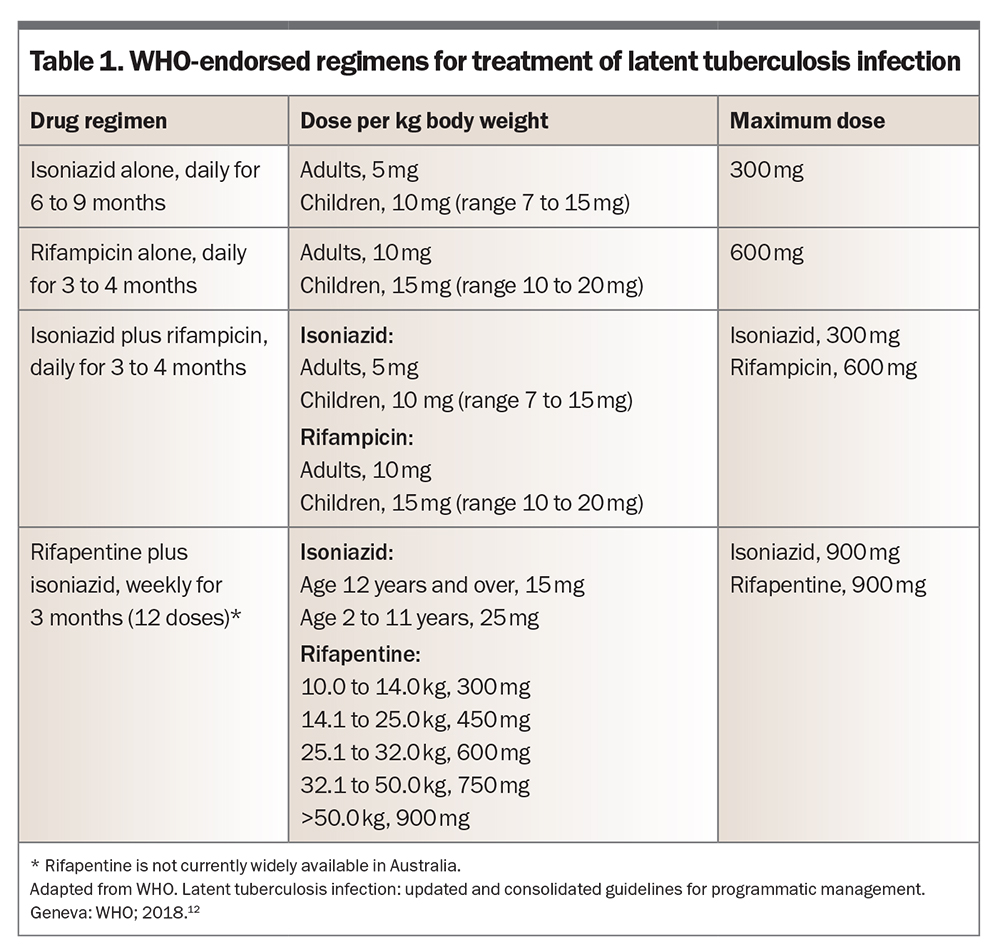
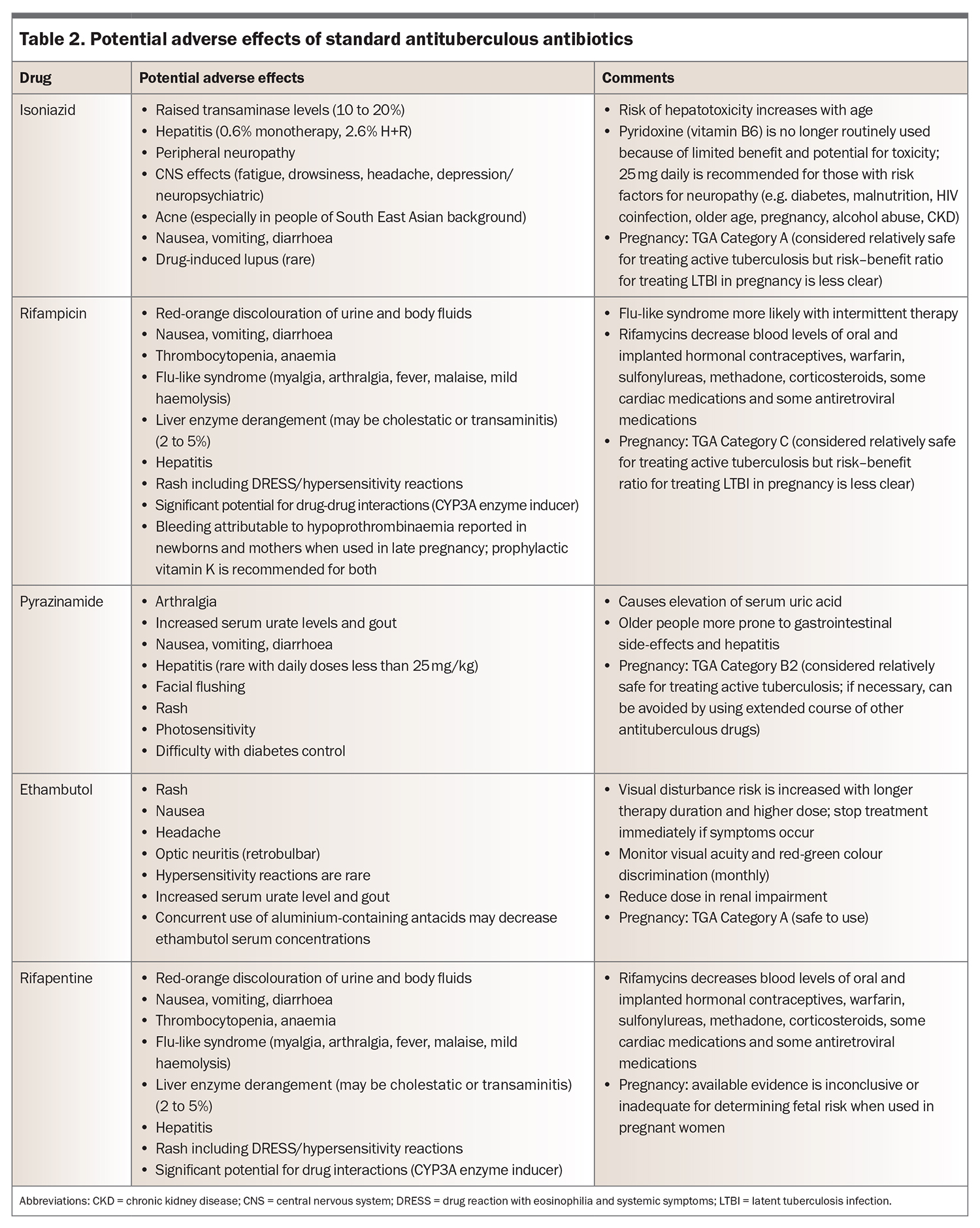
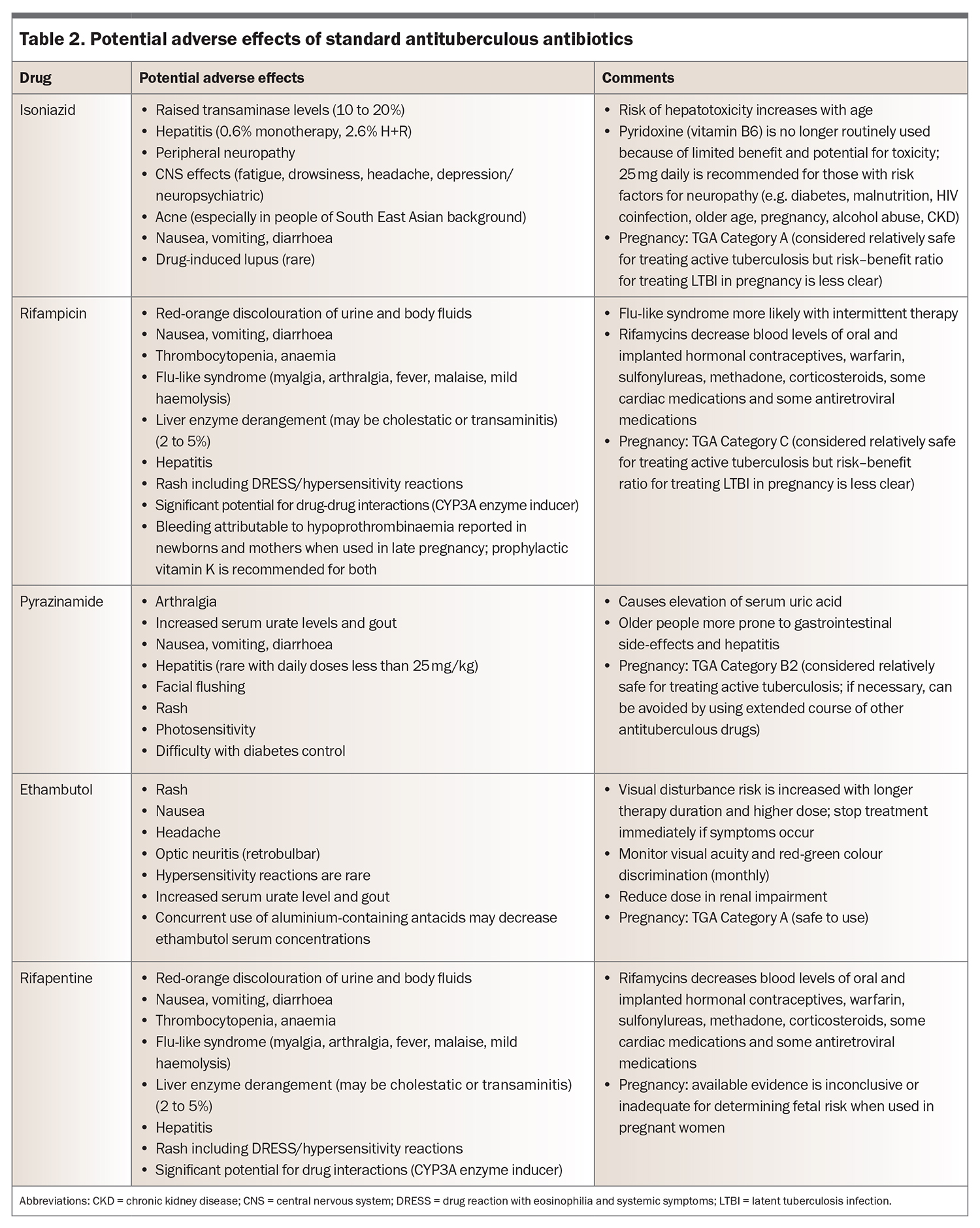
Options for evidence-based chemoprophylaxis regimens endorsed by the WHO are listed in Table 1.12 Note that rifapentine is not yet widely available for use in Australia. Potential adverse effects of isoniazid, rifampicin and rifapentine are listed in Table 2.
The most commonly prescribed chemoprophylaxis regimens are four-month daily rifampicin monotherapy (4R) and three-month combination therapy with daily rifampicin and isoniazid (3HR). Reasons for these choices include their shorter duration, which is usually preferred by patients, and lower rates of hepatotoxicity compared with six to nine months of isoniazid monotherapy.
Adverse effects of these two regimens were similar in clinical trials, although isoniazid tends to be avoided in older patients because of the increased risk of hepatotoxicity in this group. Pill burden is also a consideration; standard dosing for a patient weighing over 50 kg involves five pills daily for 3HR compared with two for 4R. Isoniazid chemoprophylaxis is still required when drug interactions are a problem, especially in patients with immunosuppression, transplantation or antiretroviral therapy.
Assessment for active tuberculosis
Investigations to order
If a patient is suspected to have active tuberculosis based on symptoms and signs, it is important to request targeted samples for AFB smear and culture. Patients with suspected pulmonary tuberculosis should be encouraged to expectorate sputum samples for testing. Sputum AFB testing is quick and inexpensive, and it is surprising how often this simple test can yield results even in the absence of a cough. The sensitivity and positive predictive value of a sputum AFB smear alone are about 45 to 80% and 50 to 80%, respectively.32,33
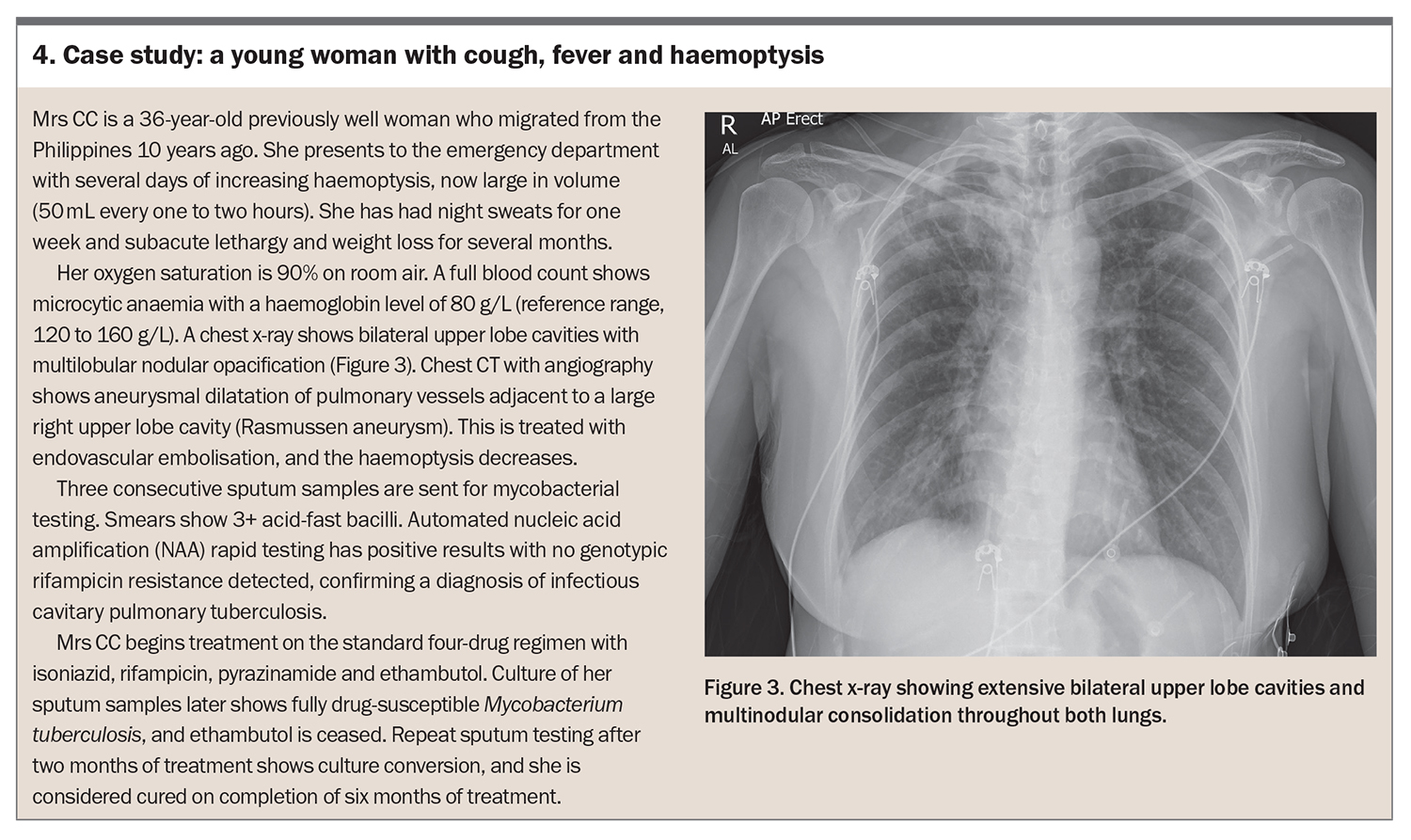
A chest x-ray is important to determine whether there are radiological changes. Typically, these include nodular opacities, cavities (particularly involving the upper lobes), pleural or pericardial effusion and hilar lymphadenopathy (see case study in Box 4).34,35 Be aware that patients who are immunosuppressed are more likely to present with disseminated disease.36-38 A CT scan is rarely required as part of routine work up for pulmonary tuberculosis but should be considered if extrapulmonary symptoms are present.
Biopsy or fluid samples to investigate suspected tuberculosis should be sent to the laboratory in saline rather than formalin for AFB smear and culture. Tuberculosis testing can be performed on a wide range of sample types, including urine, synovial and cerebrospinal fluid, and biopsy specimens from lymph nodes, the gastrointestinal tract and peritoneal and pleural tissue.39 Granulomas seen in histopathology samples should prompt consideration of tuberculosis as a potential diagnosis.
Diagnosis of active tuberculosis
The diagnosis of tuberculosis is made on culture of M. tuberculosis or detection of M. tuberculosis by nucleic acid amplification (NAA). Conventional culture is the most sensitive way to detect mycobacteria, with a sensitivity and specificity of 80% and 98%, respectively.40,41 Sputum culture can detect as few as 10 bacteria per millilitre. This can be a slow process as culturing the organism may take up to six to 10 weeks, although the average time to detection ranges from 11 to 25 days depending on the culture method.42,43 The advantage of culturing M. tuberculosis from a sample is that it allows drug susceptibility testing to guide management.
Automated NAA rapid test systems are available that can detect both M. tuberculosis complex and the rpoB gene mutation, which suggests rifampicin resistance, and give results within two hours.39,44 Most laboratories perform an automated NAA rapid test on any new AFB smear-positive specimen. This test should also be requested for AFB smear-negative samples when there is a high degree of suspicion for active tuberculosis disease. The presence of the rpoB mutation suggests rifampicin resistance, which is an initial proxy for multidrug resistance.45 Detecting genotypic rifampicin resistance at diagnosis allows earlier introduction of second-line antituberculous medications.46
Automated NAA rapid testing is highly sensitive and specific for tuberculosis. The test performs better for AFB smear-positive samples, with a sensitivity and specificity of 95 to 98% and 98%, respectively, compared with 75 to 90% and 98 to 99% for AFB smear-negative samples.32,47-50 Because of this high sensitivity, a negative NAA result for an AFB smear-positive sample is reassuring and more likely to represent a nontuberculous mycobacterial organism.
Be aware that NAA tests for M. tuberculosis may give positive results in patients with previously treated tuberculosis because of residual genetic material.
Management of active tuberculosis
When a diagnosis of active tuberculosis is confirmed, baseline investigations should include HIV testing in all patients and hepatitis B and C testing in those with epidemiological risk factors. Treatment requires multidrug therapy with a patient-centred multidisciplinary approach. Treatment is commenced as per local guidelines, which are typically based on the WHO Consolidated Guidelines on Tuberculosis.51-53
Standard therapy for uncomplicated drug-susceptible pulmonary tuberculosis and lymphadenitis commences with four drugs: isoniazid, rifampicin, ethambutol and pyrazinamide. Recommended doses are shown in Table 3.54 The medications are rationalised when drug susceptibility testing results become available, with ethambutol ceased as soon as fully drug-susceptible disease is confirmed. Pyrazinamide is then ceased after two months of treatment, completing the ‘intensive’ phase, after which the disease burden is typically significantly reduced. Following this, the patient moves to the continuation phase with isoniazid and rifampicin given for four months, to complete six months of total therapy.51 Longer courses are required for severe pulmonary infection, tuberculous meningitis, vertebral osteomyelitis, disseminated disease or drug-resistant strains.
Prompt initiation of treatment is important to reduce the transmission of M. tuberculosis.55 It is widely theorised that a patient’s infectious potential is significantly reduced after two weeks of effective tuberculosis-directed treatment, although there are no studies directly evaluating the time to reduction in transmission.55,56 Case management is an integral part of patient care and involves treatment support (a term that has replaced the legacy term of ‘directly observed therapy’ in the latest WHO consolidated guidelines), contact tracing and guidance through isolation requirements as part of public health management.57 Contrary to popular thought, AFB smear-negative patients can transmit the disease, albeit at lower rates than smear-positive patients.58 Standard advice on isolation requirements takes into consideration smear burden at diagnosis and recommends use of a surgical mask outdoors and avoidance of new contacts and young children until disease burden has reduced on therapy. Generally, AFB smear negative patients can cease isolation after two weeks of effective therapy.
Drug-resistant tuberculosis has higher mortality and is more difficult to treat than drug-susceptible disease because of the requirement for second-line agents with significant drug toxicities and longer duration.1,52 However, new regimens that include the novel agents bedaquiline, pretomanid, linezolid and fluoroquinolones have recently been endorsed by the WHO and can significantly shorten the duration of treatment of multidrug-resistant tuberculosis.52,59 Regimens are complicated and beyond the scope of this article. Any patient with drug-resistant tuberculosis must be referred to a specialist tuberculosis centre for expert management.
Patients receiving treatment for active tuberculosis undergo regular clinical reviews to assess progress and medication adherence and to monitor for adverse effects (Table 2). Liver function tests are performed at baseline, after three to four weeks of treatment and intermittently thereafter depending on risk factors for hepatotoxicity. The GP is a key member of the treating team and can assist with patient support and adherence, as well as prompt investigation of treatment-related complications. Repeat chest x-ray and sputum culture are performed after two months of treatment to assess for radiological response and culture conversion of sputum. A delayed response to treatment or poor adherence necessitate an extended course (nine months or longer), because of the risk of treatment failure and relapse.
Prognosis and review of active tuberculosis
Untreated active tuberculosis disease has a mortality rate of 50%.1 However, cure rates for uncomplicated, treatment-supported drug-susceptible tuberculosis are high, in the order of over 98% with guideline-directed therapy.60 After treatment, patients are typically reviewed at six to 12 months with a symptom check, physical examination and chest x-ray, to monitor for relapsed disease. Patients with any complications or drug resistance are monitored for a longer period after treatment. The risk of relapse is highest in the first one to two years after completion of tuberculosis therapy.61,62
Conclusion
Tuberculosis is an ongoing global issue and Australia is not impervious to its effects. Active tuberculosis disease does not discriminate and can involve any organ system, and GPs should be aware of its various manifestations. Further, latent infection is more common, and its identification and management are essential for reducing active disease and controlling spread of the infection. Tuberculosis screening tests do not differentiate between active disease and latent infection, and results can remain positive indefinitely despite treatment. Molecular testing such as NAA has revolutionised diagnosis of tuberculosis, providing both a rapid turnaround time and guidance on initial empirical therapy. Mycobacterial testing can be performed on a wide range of samples. Culture remains the gold standard for diagnosis; however, adjuncts such as AFB smear testing, histopathology and NAA are important companion tools.
With adequate, appropriate therapy, cure rates remain above 98% for susceptible disease, although patients should be monitored for adherence, adverse effects and response to treatment. As tuberculosis infection is present in our community, it is important for GPs to consider the diagnosis, particularly in populations at high risk of exposure or reactivation. RMT
COMPETING INTERESTS: None.
References
1. WHO. Global tuberculosis report 2022. Geneva: WHO: 2022. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed March 2023).
2. Drain PK, Bajema KL, Dowdy D, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018; 31: e00021-18.
3. Migliori GB, Ong CWM, Petrone L, D’Ambrosio L, Centis R, Goletti D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe (Sheff) 2021; 17: 210079.
4. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015; 372: 2127-2135.
5. Barry CE 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009; 7: 845-855.
6. Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection-the promise and the challenges. Int J Infect Dis 2017; 56: 68-76.
7. Esmail H, Macpherson L, Coussens AK, Houben R. Mind the gap - managing tuberculosis across the disease spectrum. EBioMedicine 2022; 78: 103928.
8. Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974; 99: 131-138.
9. Vynnycky E, Fine PE. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol 2000; 152: 247-263.
10. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014; 27: 3-20.
11. Shea KM, Kammerer JS, Winston CA, Navin TR, Horsburgh CR Jr. Estimated rate of reactivation of latent tuberculosis infection in the United States, overall and by population subgroup. Am J Epidemiol 2014; 179: 216-225.
12. WHO. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: WHO; 2018. Available online at: https://www.who.int/publications/i/item/9789241550239 (accessed March 2023).
13. Bumbacea D, Arend SM, Eyuboglu F, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J 2012; 40: 990-1013.
14. WHO consolidated guidelines on tuberculosis. Module 2: screening – systematic screening for tuberculosis disease. Geneva: WHO; 2021. Available online at: https://www.who.int/publications/i/item/9789240022676 (accessed March 2023).
15. Jayes L, Haslam PL, Gratziou CG, , et al. SmokeHaz: systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest 2016; 150: 164-179.
16. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis - tests for tuberculosis infection. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2022. Available online at: https://www.who.int/publications/i/item/9789240056084 (accessed March 2023).
17. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006; 10: 1192-1204.
18. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149: 177-184.
19. Zhou G, Luo Q, Luo S, et al. Interferon-gamma release assays or tuberculin skin test for detection and management of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2020; 20: 1457-1469.
20. Diel R, Goletti D, Ferrara G, et al. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011; 37: 88-99.
21. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon-gamma release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest 2012; 142: 63-75.
22. Landry J, Menzies D. Preventive chemotherapy. Where has it got us? Where to go next? Int J Tuberc Lung Dis 2008; 12: 1352-1364.
23. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41: 140-156.
24. Velen K, Shingde RV, Ho J, Fox GJ. The effectiveness of contact investigation among contacts of tuberculosis patients: a systematic review and meta-analysis. Eur Respir J 2021; 58(6) 2100266.
25. Marks GB, Bai J, Simpson SE, Sullivan EA, Stewart GJ. Incidence of tuberculosis among a cohort of tuberculin-positive refugees in Australia. Am J Resp Crit Care Med 2000; 162: 1851-1854.
26. McBryde ES, Denholm JT. Risk of active tuberculosis in immigrants: effects of age, region of origin and time since arrival in a low-exposure setting. Med J Aust 2012; 197: 458-461.
27. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA 2016; 316: 962-969.
28. Menzies D, Gardiner G, Farhat M, Greenaway C, Pai M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int J Tuberc Lung Dis 2008; 12: 498-505.
29. Molhave M, Wejse C. Historical review of studies on the effect of treating latent tuberculosis. Int J Infect Dis 2020; 92S: S31-S36.
30. Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med 2017; 167: 248-255.
31. Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174: 935-952.
32. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000; 161(4 Pt 1): 1376-1395.
33. Dinnes J, Deeks J, Kunst H, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess 2007; 11: 1-196.
34. Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics 2007; 27: 1255-1273.
35. Alshoabi SA, Almas KM, Aldofri SA, et al. The diagnostic deceiver: radiological pictorial review of tuberculosis. Diagnostics (Basel) 2022; 12(2): 306.
36. Lichtenstein IH, MacGregor RR. Mycobacterial infections in renal transplant recipients: report of five cases and review of the literature. Rev Infect Dis 1983; 5: 216-226.
37. Qunibi WY, al-Sibai MB, Taher S, et al. Mycobacterial infection after renal transplantation - report of 14 cases and review of the literature. Q J Med 1990; 77: 1039-1060.
38. Aguado JM, Herrero JA, Gavalda J, et al. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation 1997; 63: 1278-1286.
39. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection (2021 update). WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2021.
40. Morgan MA, Horstmeier CD, DeYoung DR, Roberts GD. Comparison of a radiometric method (BACTEC) and conventional culture media for recovery of mycobacteria from smear-negative specimens. J Clin Microbiol. 1983; 18: 384-388.
41. Ichiyama S, Shimokata K, Takeuchi J. Comparative study of a biphasic culture system (Roche MB Check system) with a conventional egg medium for recovery of mycobacteria. Aichi Mycobacteriosis Research Group. Tuber Lung Dis 1993; 74: 338-341.
42. Gil-Setas A, Torroba L, Fernandez JL, Martinez-Artola V, Olite J. Evaluation of the MB/BacT system compared with Middlebrook 7H11 and Lowenstein-Jensen media for detection and recovery of mycobacteria from clinical specimens. Clin Microbiol Infect 2004; 10: 224-228.
43. Hanna BA, Ebrahimzadeh A, Elliott LB, et al. Multicenter evaluation of the BACTEC MGIT 960 system for recovery of mycobacteria. J Clin Microbiol 1999; 37: 748-752.
44. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTF/RIF Ultra compared to Xpert MTB/RIF. Geneva: WHO; 2017. Available online at: https://www.who.int/publications/i/item/WHO-HTM-TB-2017.04 (accessed March 2023).
45. Shinnick TM, Starks AM, Alexander HL, Castro KG. Evaluation of the Cepheid Xpert MTB/RIF assay. Expert Rev Mol Diagn 2015; 15: 9-22.
46. Ershova JV, Volchenkov GV, Somova TR, et al. Impact of GeneXpert MTB/RIF(R) on treatment initiation and outcomes of RIF-resistant and RIF-susceptible TB patients in Vladimir TB dispensary, Russia. BMC Infect Dis 2020; 20: 543.
47. Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011; 377: 1495-1505.
48. Guerra RL, Hooper NM, Baker JF, et al. Use of the amplified mycobacterium tuberculosis direct test in a public health laboratory: test performance and impact on clinical care. Chest 2007; 132: 946-951.
49. Marks SM, Cronin W, Venkatappa T, et al. The health-system benefits and cost-effectiveness of using Mycobacterium tuberculosis direct nucleic acid amplification testing to diagnose tuberculosis disease in the United States. Clin Infect Dis 2013; 57: 532-542.
50. Luetkemeyer AF, Firnhaber C, Kendall MA, et al. Evaluation of Xpert MTB/RIF versus AFB smear and culture to identify pulmonary tuberculosis in patients with suspected tuberculosis from low and higher prevalence settings. Clin Infect Dis 2016; 62: 1081-1088.
51. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-susceptible tuberculosis treatment. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2022. Available online at: https://www.who.int/publications/i/item/9789240048126 (accessed March 2023).
52. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2022. Available online at: https://www.who.int/publications/i/item/9789240007048
53. WHO consolidated guidelines on tuberculosis. Module 5: management of tuberculosis in children and adolescents. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2022. Available online at: https://www.who.int/publications/i/item/9789240046764 (accessed March 2023).
54. Communicable Diseases Branch, Queensland Health. Treatment of tuberculosis in adults and children - guideline Version 3.0. Brisbane: State of Queensland (Queensland Health); 2021. Available online at: https://www.health.qld.gov.au/__data/assets/pdf_file/0029/444566/tb-guideline-treatment.pdf (accessed March 2023).
55. WHO consolidated guidelines on tuberculosis. Module 1: prevention - infection prevention and control. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2022. Available online at: https://apps.who.int/iris/bitstream/handle/10665/362508/9789240055889-eng.pdf (accessed March 2023).
56. WHO guidelines on tuberculosis infection prevention and control: 2019 update. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2019. Available online at: https://www.who.int/publications/i/item/9789241550512 (accessed March 2023).
57. WHO consolidated guidelines on tuberculosis. Module 4: treatment - tuberculosis care and support. WHO guidelines approved by the Guidelines Review Committee. Geneva: WHO; 2022. Available online at: https://www.who.int/publications/i/item/9789240047716 (accessed March 2023).
58. Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999; 353: 444-449.
59. Nyang’wa BT, Berry C, Kazounis E, et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N Engl J Med 2022; 387: 2331-2343.
60. Five-year follow-up of a controlled trial of five 6-month regimens of chemotherapy for pulmonary tuberculosis. Hong Kong Chest Service/British Medical Research Council. Am Rev Respir Dis 1987; 136: 1339-1342.
61. Shao Y, Song H, Li G, et al. Relapse or re-infection, the situation of recurrent tuberculosis in Eastern China. Front Cell Infect Microbiol 2021; 11: 638990.
62. Youn HM, Shin MK, Jeong D, Kim HJ, Choi H, Kang YA. Risk factors associated with tuberculosis recurrence in South Korea determined using a nationwide cohort study. PLoS One 2022; 17: e0268290.